- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Xeomin approved for treatment of Chronic Sialorrhea in Adults
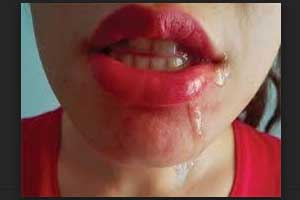
U.S Food and Drug Administration(FDA) approved the supplemental Biologics License Application (sBLA) for Xeomin (incobotulinumtoxinA) for the treatment of chronic sialorrhea in adult patients, announced, Merz Neurosciences.
Sialorrhea is a common symptom among patients who suffer from neurological disorders including Parkinson's disease, amyotrophic lateral sclerosis (ALS), cerebral palsy (CP) or who have had a stroke. The condition can occur from difficulty retaining saliva inside the mouth, issues with swallowing and from problems controlling facial muscles.
Xeomin is an acetylcholine release inhibitor and neuromuscular blocking agent indicated for the treatment or improvement of adult patients with:
• chronic sialorrhea
• upper limb spasticity
• cervical dystonia
• blepharospasm with onabotulinumtoxinA (Botox) prior treatment
• temporary improvement in the appearance of moderate to severe glabellar lines with corrugator and/or procerus muscle activity
The FDA approved Xeomin, supplemental Biologics License Application (sBLA) for Xeomin after granting the application a priority review designation. Priority reviews are given to drugs that will potentially provide significant improvements in the safety and effectiveness of the treatment, diagnosis or prevention of serious conditions.
The recommended total dose for Chronic Sialorrhea is 100 Units per treatment session consisting of 30 Units per parotid gland and 20 Units per submandibular gland, no sooner than every 16 weeks.
Xeomin bagged the approval after Phase 3, randomized, double-blind, placebo-controlled, multicenter (N=184) trial. The study involved participants who had chronic sialorrhea for ≥3 months were assigned to placebo, Xeomin 75 Units, or Xeomin 100 Units. The study found that there was a statistically significant improvement in the change in unstimulated Salivary Flow Rate (uSFR) and Global Impression of Change Scale (GICS) at week 4 vs baseline pre-injection for patients in the Xeomin 100 Units group. Xeomin 75 Units did not perform significantly better than placebo.
The most common side effects of Xeomin in people with chronic sialorrhea include needing to have a tooth pulled (extracted), dry mouth, diarrhea, and high blood pressure. The drug is available as 50 Units, 100 Units, and 200 Units lyophilized powder in single-dose vials.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd