- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Two common uveitis drugs found equally effective in noninfectious uveitis in clinical trial: JAMA
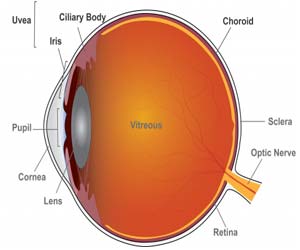
Methotrexate and mycophenolate mofetil performed similarly in a head-to-head clinical trial that compared the two drugs for treating noninfectious uveitis, an eye disease that accounts for up to 15% of blindness in the U. S. In cases of more severe disease, posterior uveitis, and panuveitis, the international trial showed that methotrexate was more effective in controlling inflammation.The results from the trial have been published in the Journal of the American Medical Association.
“This study gives doctors and their patients with uveitis a starting point when considering treatment beyond corticosteroids,” said lead study author Nisha Acharya, M.D., M.S., University of California, San Francisco.
Uveitis is inflammation of the eye’s blood vessel-rich middle layer of tissue called the uvea. The condition can affect the iris (anterior uveitis), ciliary body (intermediate uveitis), and choroid (posterior uveitis) parts of the eye and is often chronic. Panuveitis affects multiple areas of the uvea. Clinicians often first treat intermediate and posterior or panuveitis with oral corticosteroids like prednisone to control inflammation, but seek to quickly taper patients to a low dose and switch them to steroid-sparing drugs such as methotrexate and mycophenolate mofetil. Long-term, high-dose corticosteroid use risks serious side effects including osteoporosis, diabetes, and weight gain, and other eye problems including glaucoma and cataract. Other steroid alternatives such as biologics like adalimumab also carry serious side effects.
The First-line Antimetabolites for Steroid-sparing Treatment (FAST) Uveitis Trial enrolled and randomly assigned 216 patients with intermediate or posterior/panuveitis from India, the United States, Australia, Saudi Arabia and Mexico to methotrexate (107 participants) or mycophenolate (109 participants) treatment groups. Over six months, participants tapered to a maximum dose of 7.5 milligrams prednisone daily, while receiving either three grams oral mycophenolate daily or 25 milligrams methotrexate weekly. Participants reduced their dose, if necessary, to control adverse side effects such as nausea.
To assess control of inflammation, the research team performed clinical exams and ocular imaging in the front and back parts of the eye. They also checked visual acuity.
At six months, 67% of participants in the methotrexate group and 57% of participants in the mycophenolate group had controlled their inflammation and successfully tapered steroids. The remainder had the option to switch treatments. Differences in success rates between treatment groups were not statistically significant at six months. At 12 months, 69% of participants who had switched from mycophenolate to methotrexate achieved treatment success, whereas only 35% of those participants who switched from methotrexate to mycophenolate achieved treatment success. Of the participants who continued with their original treatments, 80% on methotrexate and 74% on mycophenolate maintained inflammatory control at 12 months.
In patients with posterior or panuveitis, the most severe forms, 74% in the methotrexate group achieved control at six months, versus 55% in the mycophenolate group, indicating that methotrexate was significantly more effective at controlling inflammation for this subtype of uveitis.
Both methotrexate and mycophenolate mofetil, which are systemic and affect multiple cell types in the body, can cause side effects such as fatigue, nausea and headaches, but serious side effects are rare. Because participants were allowed to reduce dosage to control these side-effects, few of them dropped out of the FAST study due to their inability to tolerate the medication. Total rates of adverse events were similar between the two drugs.
“Based on this head-to-head clinical trial, methotrexate is as good as or better than mycophenolate for treating uveitis. That’s important because the prior literature and a survey on treatment preferences suggests most clinicians believe the opposite. Now we have a randomized trial to provide guidance on treatment.” said Acharya. “Additionally, there’s a cost difference in the U.S. where mycophenolate to control uveitis is over five times more expensive.”

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd