- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Topical JAK Inhibitor - Future treatment for Alopecia Areata
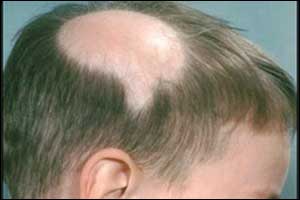
U.S. Food and Drug Administration (FDA) has granted Fast Track designation to an investigational topical Janus Kinase (JAK) 1/3 inhibitor, ATI-502 for the treatment of alopecia areata, including patchy alopecia areata and the more severe variants of the disease, announced Aclaris Therapeutics, Inc, a dermatologist-led biopharmaceutical company.
ATI-502 is under investigation for alopecia areata and more severe variants, alopecia totalis, and Universalis. The company had announced positive interim data from a Phase 2 study (AA-202 Topical) in June 2018, which showed that ATI-502 is absorbed through human skin and targets the genes implicated in alopecia areata.
JAK signaling has shown to maintain the hair cycle in its resting phase (telogen) in mice. Treatment of mouse telogen skin with topical JAK inhibitors prompts telogen follicles to enter the active growth phase (anagen). Hair loss disorders in which the hair follicles are arrested in telogen phase may be responsive to treatment with topical JAK inhibitors to promote their entry into anagen, and thus result in the initiation of hair growth.
Alopecia areata is an autoimmune disease that results in partial or complete loss of hair on the scalp and body. The scalp is the most commonly affected area, but any hair-bearing site can be affected alone or together with the scalp. It usually starts with one or more small, round, smooth patches. It occurs in males and females of all ages and races, but onset most often occurs in childhood. AA affects up to 2.0% of people globally at some point during their lifetime (i.e. incidence) and up to 0.2% of people are affected at any given time (i.e. prevalence). There are currently no drugs approved by the FDA for the treatment of AA.
"We look forward to working closely with the FDA throughout our development program with the hope of ultimately bringing this important treatment option to patients," said Christopher Powala, Chief Regulatory & Development Officer of Aclaris.
Fast track is a process designed to facilitate the development, and expedite the review of drugs to treat serious conditions and fill an unmet medical need. The purpose is to get important new drugs to the patient earlier. Fast Track addresses a broad range of serious conditions.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd