- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Squirtable Surgical Glue Could Transform Surgeries and Save Lives
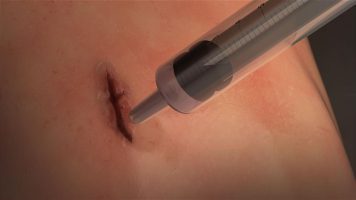
Sutures and staples are the traditional methods for closing surgical incisions and wounds in emergency situations. However, these methods can be inadequate in complex surgeries and cannot make an air-tight or liquid-tight seal on a lung or artery wound or incision. Now researchers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have created a surgical glue that is squirted onto wounds and then sets to form an elastic air-tight or liquid-tight seal in just one minute. Successfully tested in animals, the sealant has enormous promise for life-saving use in humans.
A team of researchers led by Ali Khademhosseini, Ph.D., Professor, Departments of Radiology and Chemical and Biomolecular Engineering at the University of California Los Angeles has developed a biocompatible hydrogel elastic sealant that has the potential to replace the use of sutures and staples to close wounds, and to do it more rapidly and effectively.
The compound, which can be formulated to work optimally in different situations, is a gel-like substance called methacryloyl-substituted tropoelastin (MeTro). The development and testing of MeTro in animal models are reported in Science Translational Medicine.
“There are a number of surgical sealants currently available,” said David Rampulla, Ph.D., Director of the NIBIB Program in Biomaterials, “however, they have limited uses because of problems like dissolving when in contact with bodily fluids. The remarkable thing about MeTro is that it can be fine-tuned to hold up in demanding situations. For instance, when surgeons repair a cut artery, the sealant can maintain a liquid-tight bond as it comes in contact with blood that’s under pressure as it pumps through the artery.”
MeTro has a number of physical qualities that make it extremely versatile. As a relatively thick liquid it can be squirted onto an incision or tissue tear, and will adhere to, and flow into the wound to make a tight seal. The researchers designed it so that by applying UV light, MeTro will solidify in place, but remain flexible enough to expand and contract with the movement of the repaired organ, such as the expansion and contraction of a repaired lung.
The MeTro formulation can also be changed to vary the amount of time it stays on the wound. The formula can be tweaked so that it remains for as long as it takes the wound to heal, and then dissolves; this can be days or months, depending on the wound-healing requirements. The ability of MeTro to dissolve after its work is done is an extremely useful quality, because other sealants that do not dissolve are known to cause irritation and promote infection.
So far, MeTro has been tested in animal models and has been used to successfully seal experimental incisions in the arteries of rodents. It also performed well in experiments sealing experimental lung incisions in both rodents and pigs.
“We are now ready to begin testing in humans,” said Khademhosseini. “We look forward to seeing MeTro saving lives in the clinic, operating room, and in emergency situations.”
Additional senior scientists co-leading the international collaboration with Khademhosseini are Anthony S. Weiss, Ph.D., Professor of Biochemistry and Molecular Biotechnology, School of Life and Environmental Sciences, University of Sydney, Australia, and Nasim Annabi, Ph.D., Assistant Professor of Chemical Engineering, Northeastern College of Engineering, Boston, Massachusetts.
The work was supported by grants EB023052, EB022043, EB021148, and EB014283 from the National Institute of Biomedical Imaging and Bioengineering, and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of Dental and Craniofacial Research at the National Institutes of Health. Additional funding was provided by the Australian Research Council, the American Heart Association, Interdisciplinary Research Seed Grants from Northeastern University, and the German Heart Foundation.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd