- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Smallest sized replacement heart valve approved in the world
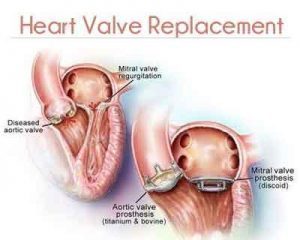
The U.S. Food and Drug Administration has approved Masters HP™ 15mm rotatable mechanical heart valve which is the smallest mechanical heart valve approved in the world.This dime-sized new valve is the first and only pediatric mechanical heart valve developed for newborns and infants and offers hope for pediatric patients in urgent need of treatment who have no other approved options.
Heart valve disease occurs if one or more of the four heart valves, which direct the flow of blood through the heart, fail to function properly. In pediatric patients, a malfunctioning heart valve is often the result of a congenital heart defect at birth. Each year, more than 35,000 babies in the U.S. are born with congenital heart defects, some of which will require heart valve surgery and, potentially, replacement heart valve surgery. However, prior to today’s approval, there have been limited replacement heart valve options available because of the patients’ small size. The Masters Series 15-mm HP valve represents an important treatment option for these patients.
“While larger replacement heart valves have been approved for years, there is an unmet need in young pediatric patients, especially newborns and infants, with congenital valve defects who may be too small to use currently-marketed heart valves,” said Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health.
The Master Series Mechanical Heart Valve is a rotatable, bileaflet (two-leaflet) valve designed for implantation in the aortic or mitral position. The bileaflet design consists of two semi-circular discs that open and close in response to blood pressure changes during the heartbeat, similar to a patient’s own valve.
The Masters Series Mechanical Heart Valve was first approved in 1995 for patients with a diseased, damaged or malfunctioning aortic or mitral heart valve. The device is also approved for use in replacing previously implanted aortic or mitral prosthetic heart valves. Today’s approval expands the range of valve sizes available, providing smaller patients another treatment option.
The FDA evaluated clinical data from a single-arm study of 20 pediatric patients with serious heart failure ranging in age from 1.5 weeks to 27 months at the time of mitral valve implant. The data showed that one year after the implant procedure, the probability of survival was 69.3 percent and the probability of not experiencing a valve-related adverse event was 66.8 percent. Serious valve-related adverse events observed during the study through one-year follow-up included blood clots in the device and bleeding in the brain. Anticoagulation (blood thinning) therapy may be necessary after the procedure, to prevent clotting on the device, which can increase the risk of bleeding.
The Master Series Mechanical Heart Valve should not be used by patients unable to tolerate anticoagulation therapy.
The FDA granted approval of the Master Series Heart Valve to St. Jude Medical.
The FDA, an agency within the U.S. Department of Health and Human Services, promotes and protects the public health by, among other things, assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd