- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Researchers develop non-invasive optical imaging system to improve treatment for dry eye disease
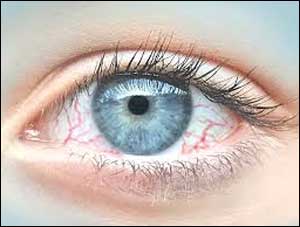
Tears are necessary for maintaining the health of the eye and for providing clear vision. Dry eye is a common and often chronic condition in which a person doesn't have enough quality tears to lubricate and nourish the eye. Dry eye often causes irritation and blurred vision, when there is instability in the inner layer of the tear film that protects the outside of the eye.
The usual treatment of dry eye comprises of Eyedrops and ointments that keep the lubrication of eye intact by forming an artificial layer of tears. Now researchers have developed a new non-invasive optical imaging system that promises to improve diagnosis and treatments for dry eye disease. The researchers describe the device's ability to perform spectral measurements across a large field of view in a matter of seconds. They report that the imager can acquire fast and consistent measurements from human eyes even when blinking. The details of research have been published in the Optical Society (OSA) journal Applied Optics.
"Up to 60 per cent of ophthalmology office visits are due to dry eye, pointing to the need for a non-invasive and highly accurate device for diagnosis in the office setting," said research team leader Dr Yoel Arieli from AdOM Advanced Optical Methods Ltd. in Israel. "Our Tear Film Imager is the first device that can be used in the ophthalmology or optometry setting to image the tear film and distinguish its inner layers with nanometer resolution."
Today, most cases of dry eye are diagnosed using patient questionnaires, which may be subjective and cannot typically be used to identify the cause of the disease. Objective methods for examining the tear film tend to be invasive and cannot track rapid changing dynamics, which are altered with every blink.
The research study was performed at AdOM Advanced Optical Methods laboratory. The proof of concept, unique method and design that enable measurement of ultra-thin tear layers with nanometer resolution were developed by AdOM's development team led by Dr. Yoel Cohen.
"For the diagnosis of dry eye disease, there have been few significant advancements over recent years," said Dr Arieli. "We collaborated with academic and practising physicians who diagnose and treat dry eye to develop an instrument that can be integrated into a clinical setting while very accurately imaging the tear film inner layers, which can be used to diagnose dry eye and understand its cause."
The new instrument uses an eye-safe halogen light to illuminate the eye and then analyzes the full spectrum of light reflection over time and space. These spectral measurements are used to reconstruct the structures found in the front of the eye, allowing accurate measurement of the tear film inner layers, especially the aqueous sublayer. This sublayer plays an important role in dry eye but has been difficult to analyze with other methods.
"The broadband illumination source and fine details available from spectral analysis provide nanometer-level insight into subtle changes in each tear film layer and sublayer," said Dr. Arieli. "These measurements are completed automatically in just 40 seconds."
After demonstrating a resolution of 2.2 nanometers on a mock tear film, the researchers tested the instrument's ability to take measurements on the human eye with no intervention while the patient was blinking. "The device worked impressively and presents no risk because it is non-invasive and uses a simple light source," said Dr. Arieli. "It not only measured the tear film consistently including blinks every few seconds, but the measurements correlated well with other partially invasive, established dry eye diagnostic techniques."
The Tear Film Imager has been used in two clinical studies in Israel and Canada, examining dry eye diagnosis with the device and dry eye treatments, which can be precisely evaluated with the imager. The researchers say that future studies could help inform better dry eye treatments, improve surgical outcomes and lead to more accurate fitting of contact lenses. They are also planning to use the Tear Film Imager in larger studies with more diverse groups of patients to set base levels for both healthy eyes and people with dry eye.
Disclaimer: The TFI and the information provided about it have not been evaluated by the U.S. Food and Drug Administration. The TFI has yet to receive any regulatory approvals.
For further reference log on to :
Applied Optics, 2019; 58 (29): 7987 DOI: 10.1364/AO.58.007987

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd