- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
PVP-I/dexamethasone OS Effective for Treatment of Adenoviral Conjunctivitis- Phase 2 Trial
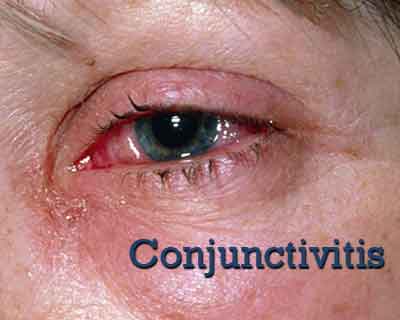
Povidone-Iodine/Dexamethasone appeared to be safe and effective in patients with acute adenoviral conjunctivitis, according to a study published in American Journal of Opthalmology.
Around 79% of patients got relief from Adeno conjunctivitis when treated with PVP-I/dexamethasone as compared to 62% when treated with PVP-I. Viral conjunctivitis is a highly contagious acute conjunctival infection usually caused by adenovirus. Symptoms include irritation, photophobia, and watery discharge.
Jay S Pepose and his associates conducted a study to evaluate the efficacy and safety of an ophthalmic suspension of povidone-iodine (PVP-I) 0.6% and dexamethasone 0.1% in patients with acute adenoviral conjunctivitis.
The trial conducted was multicentered, randomized, vehicle-controlled, double-masked which involved 144 patients with a positive Rapid Pathogen Screening Adeno-Detector Plus TM test. The patients were randomly given PVP-I 0.6%/dexamethasone 0.1%, PVP-I 0.6%, or vehicle in equal proportion 1:1:1 bilaterally 4 times daily for 5 days. Patients were evaluated on days 3, 6, and 12.
The study found that the proportion with adenoviral eradication was higher with PVP-I/dexamethasone than vehicle at the day 3 (35.4% vs 8.7% ) and day 6 (79.2% vs 56.5%) visits and versus PVP-I (day 3 visit, 32.0%; day 6 visit, 62.0%).
Treatment-emergent adverse events (AEs) occurred in 69.0% (vehicle), 62.7% (PVP-I), and 53.4% (PVP-I/dexamethasone) of patients.
The study concluded that PVP-I/dexamethasone appeared safe and well tolerated, and significantly improved clinical resolution and adenoviral eradication in patients with acute adenoviral conjunctivitis.
For more information log on to

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd