- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Purdue researchers reveal Zika Virus Structure

New York : US researchers have for the first time determined the structure of the Zika virus, revealing insights critical to the development of effective antiviral treatments and vaccines for the deadly disease.
Researchers from Purdue University in the US studied a strain of Zika virus isolated from a patient infected during the French Polynesia epidemic and determined the structure of the virus.
The team also identified regions within the Zika virus structure where it differs from other flaviviruses, the family of viruses to which Zika belongs that includes dengue, West Nile, yellow fever, Japanese encephalitis and tick-borne encephalitic viruses.
The structure details vital differences on a key protein that may explain why Zika attacks nerve cells while other viruses in the same family do not, the scientists maintained.
"The structure of the virus provides a map that shows potential regions of the virus that could be targeted by a therapeutic treatment, used to create an effective vaccine or to improve our ability to diagnose and distinguish Zika infection from that of other related viruses," said lead researcher Richard Kuhn from Purdue University.
The study, detailed online in the journal Science, found the structure to be very similar to that of other flaviviruses, which includes dengue, West Nile, yellow fever, Japanese encephalitis and tick-borne encephalitic viruses, with an RNA genome surrounded by a lipid, or fatty, membrane inside an icosahedral protein shell.
Prinicipal Researchers Rossmann and Kuhn collaborated with Theodore Pierson, chief of the viral pathogenesis section of the Laboratory of Viral Diseases at the National Institutes of Health National Institute of Allergy and Infectious Diseases. Additional research team members include Purdue graduate student Devika Sirohi and postdoctoral research associates Zhenguo Chen, Lei Sun and Thomas Klose.
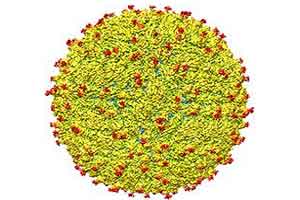 A representation of the surface of the Zika virus is shown. A team led by Purdue University researchers is the first to determine the structure of the Zika virus, which reveals insights critical to the development of effective antiviral treatments and vaccines. (Purdue University image/courtesy of Kuhn and Rossmann research groups)
A representation of the surface of the Zika virus is shown. A team led by Purdue University researchers is the first to determine the structure of the Zika virus, which reveals insights critical to the development of effective antiviral treatments and vaccines. (Purdue University image/courtesy of Kuhn and Rossmann research groups)The team found that all of the known flavivirus structures differ in the amino acids that surround a glycosylation site in the virus shell. The shell is made up of 180 copies of two different proteins. These, like all proteins, are long chains of amino acids folded into particular structures to create a protein molecule, Rossmann said.
The glycosylation site where Zika virus differs from other flaviviruses protrudes from the surface of the virus. A carbohydrate molecule consisting of various sugars is attached to the viral protein surface at this site.
In many other viruses it has been shown that as the virus projects a glycosylation site outward, an attachment receptor molecule on the surface of a human cell recognizes the sugars and binds to them, Kuhn said.
The virus is like a menacing stranger luring an unsuspecting victim with the offer of sweet candy. The human cell gladly reaches out for the treat and then is caught by the virus, which, once attached, may initiate infection of that cell.
The glycosylation site and surrounding residues on Zika virus may also be involved in attachment to human cells, and the differences in the amino acids between different flaviviruses could signify differences in the kinds of molecules to which the virus can attach and the different human cells it can infect, Rossmann said.
“If this site functions as it does in dengue and is involved in attachment to human cells, it could be a good spot to target an antiviral compound,” Rossmann said. “If this is the case, perhaps an inhibitor could be designed to block this function and keep the virus from attaching to and infecting human cells.”
“Most viruses don’t invade the nervous system or the developing fetus due to blood-brain and placental barriers, but the association with improper brain development in fetuses suggest Zika does,” Sirohi said. “It is not clear how Zika gains access to these cells and infects them, but these areas of structural difference may be involved. These unique areas may be crucial and warrant further investigation.”

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd