- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Personalizing treatment of gastrointestinal cancers with patient-derived organoids: British Journal of Surgery
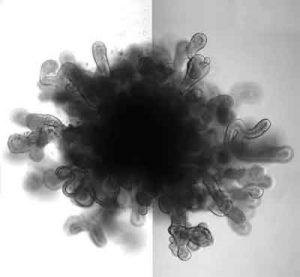
A new BJS (British Journal of Surgery) review highlights the potential of 3D organoid models derived from patient cells to help personalize therapy for individuals with gastrointestinal cancers.
Organoids--artificially grown masses of cells or tissues that resemble organs--are being used by researchers in a range of biomedical fields as they study various disease states and work to develop potential treatments.
Organoid culture methods have been established for healthy and diseased tissues from oesophagus, stomach, intestine, pancreas, bile duct, and liver. Because organoids can be generated with high efficiency and speed from patient samples, they can serve as a personal cancer model that can guide clinical decision-making.
"Research using organoids has already unraveled so many of the underlying causes of cancer. But, for me, their real promise lies in their capacity to predict treatment response," said lead author Merel Aberle, MD, of Maastricht University, in The Netherlands. "In future, organoids could be used to find the best treatment combination for every patient, thereby increasing response rates, but also reducing the side-effects unnecessary treatment may cause. I hope that this review inspires more surgeons, oncologists, radiologists, and other healthcare professionals to use this model and set up collaborative clinical trials."

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd