- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Patient develops pemphigoid lichen planus after use of diabetes drug: Case Report
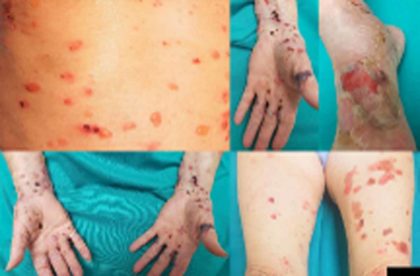
Spain: A recent case report published in the International Journal of Dermatology describes the case of an 80-year-old woman who developed blisters on her abdomen and extremities 3 months after starting therapy with vildagliptin, an oral dipeptidyl peptidase-4 inhibitor, indicated for the treatment of type 2 diabetes.
Histologic exam including direct immunofluorescence was consistent with pemphigoid lichen planus (PLP), a rare variant of lichen planus.
The woman had a personal history of Type II diabetes who was under treatment with vildagliptin 50 mg/day since April 2018. She presented a bullous rash located on the limbs and abdomen in July 2018. Blisters located on the hands showed hematic content inside and rested overnoninflammatory skin.
 Tense blisters of clear and hemorrhagic content located in abdomen, limbs, and acral areas
Tense blisters of clear and hemorrhagic content located in abdomen, limbs, and acral areasPemphigoid lichen planus is currently classified as a rare variant of lichen planus (LP) characterized by blisters located mainly on the limbs and less often in the rest of the cutaneous surface, sometimes affecting mucosae as well. Etiopathogenesis of this condition is not clearly established. Some authors defend that PLP may be considered as a variant of bullous pemphigoid (BP), and on the other hand it could be considered as a simple association of LP and BP.
First of all, the doctors interrupted vildagliptin treatment, changing it to another antidiabetic drug and starting treatment with oral prednisone (30 mg/day) until the condition was under control (meaning the stop of the appearance of new lesions and the epithelization of the old ones). Doses of prednisone under 15 mg/day were associated with the appearance of new lesions, so an immunosuppressant treatment was added. Mycophenolate mofetil (MMF) 1 g/12 hour, in addition to a daily dose of 5 mg prednisone was started with good control of the process in 3 months.
"There are 78 cases published in the literature regarding PLP. We present the first case of PLP secondary to vildagliptin. The relation between BP and gliptins and the report of our case may open the debate about whether PLP is really a variant of BP or if it should be considered a whole different entity.
To read the case in detail follow the link: doi: 10.1111/ijd.14630

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd