- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Overactive bladder drug Mirabegron does not increase CV risk: JAMA
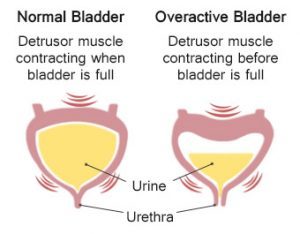
Canada: Mirabegron (Myrbetriq) -- a drug commonly prescribed for the treatment of overactive bladder (OAB) -- does not increase the risk of cardiovascular (CV) events compared to other treatments for the condition, finds a recent study published in the journal JAMA Internal Medicine. CV events included arrhythmia, tachycardia, heart attack (myocardial infarction), stroke.
The first β3-adrenoceptor agonist mirabegron has been prescribed for the treatment of overactive bladder more frequently than antimuscarinic agents. This is because the β3- agonists have fewer side effects compared to antimuscarinic agents. Owing to their ability to increase contractile force and reduce inotropic effects, the use of β3-adrenoreceptors raises concerns of cardiovascular adverse effects. This is based on the findings from trials that have associated its use with a small increase in blood pressure, heart rate and QTc intervals. However, real-world data among older patients with CV comorbidities are lacking.
Mina Tadrous, Women's College Research Institute, Women's College Hospital, Toronto, Ontario, Canada, and colleagues conducted a population-based cohort study to evaluate the risk of cardiac arrhythmias and other CV events in a population of patients 66 years and older receiving mirabegron.
For the study, the researchers evaluated Ontario's provincial healthcare administrative database 21,870 patients who first received OAB prescriptions and 16,948 patients first prescribed mirabegron from June 1, 2015, to March 31, 2017, matched for age, sex, index date, and propensity score using the Ontario's provincial healthcare administrative database. The cohort was 64.9% female and all over age 65 (median 76 years). Hypertension (30 393 [78.3%]) and diabetes (13 757 [35.4%]) were highly prevalent in the cohort.
Also Read: Scientists develop tiny, implantable device for treating overactive bladder
Key findings of the study include:
- The 1-year cumulative incidence (adjusted for person-years) of arrhythmia or tachycardia events was similar between exposure groups (3.6% for mirabegron vs 3.8% for other OAB drugs).
- Mirabegron was not associated with an increased risk of myocardial infarction (MI) or stroke compared with other OAB drugs.
- Results were seen in subgroups without prior atrial fibrillation or ventricular arrhythmia and in those ages 75 and older (2.4% for mirabegron vs 2.3%).
Also Read: Management of Urinary incontinence and pelvic organ prolapse in women: 2019 NICE Guidelines
"Our findings do not endorse preferential use of mirabegron but support the growing body of evidence that mirabegron is not associated with an excess risk of CV events compared with other treatments in older patients," write the authors.
"These results appear to support current prescribing patterns and give a balanced view of real-world safety," they concluded.
To read the complete study follow the link: doi:10.1001/jamainternmed.2019.2011

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd