- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Now auto adjustable lens after cataract surgery
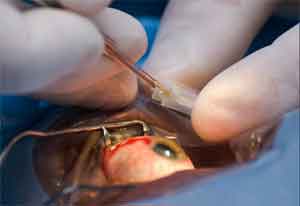
The U.S. Food and Drug Administration approved the RxSight Inc. Light Adjustable Lens and Light Delivery Device, the first medical device system that can make small adjustments to the artificial lens’ power after cataract surgery so that the patient will have better vision when not using glasses.
Cataracts are a common eye condition where the natural lens becomes clouded, impairing a patient’s vision. Following cataract surgery, during which the natural lens of the eye that has become cloudy is removed and replaced with an artificial lens ( or IOL), many patients have some minor residual refractive error requiring the use of glasses or contact lenses. Refractive error, which is caused when the artificial lens does not focus properly, causes blurred vision.
“Until now, refractive errors that are common following cataract surgery could only be corrected with glasses, contact lenses or refractive surgery,” said Malvina Eydelman, M.D., director of the Division of Ophthalmic, and Ear, Nose and Throat at the FDA’s Center for Devices and Radiological Health. “This system provides a new option for certain patients that allows the physician to make small adjustments to the implanted lens during several in-office procedures after the initial surgery to improve visual acuity without glasses.”
The RxSight IOL is made of a unique material that reacts to UV light, which is delivered by the Light Delivery Device, 17-21 days after surgery. Patients receive three or four light treatments over a period of 1-2 weeks, each lasting about 40-150 seconds, depending upon the amount of adjustment needed. The patient must wear special eyeglasses for UV protection from the time of the cataract surgery to the end of the light treatments to protect the new lens from UV light in the environment.
A clinical study of 600 patients was conducted to evaluate the safety and effectiveness of the RxSight Light Adjustable Lens and Light Delivery Device. Six months after the procedure, patients on average saw an improvement of about one additional line down the vision chart, for distance vision without glasses, compared to a conventional IOL. Six months after surgery, 75 percent also had a reduction in astigmatism.
The device is intended for patients who have astigmatism (in the cornea) before surgery and who do not have macular diseases.
The device should not be used in patients taking systemic medication that may increase sensitivity to UV light such as tetracycline, doxycycline, psoralens, amiodarone, phenothiazines, chloroquine, hydrochlorothiazide, hypercin, ketoprofen, piroxicam, lomefloxacin and methoxsalen. Treatment in patients taking such medications may lead to irreversible eye damage. The device is also contraindicated in cases where patients have a history of ocular herpes simplex virus.
The FDA approved the Vision Light Adjustable Lens and the Light Delivery Device to RxSight Inc.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd