- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
New FDA Approved Tech Moves Us Closer to a Robotic Future
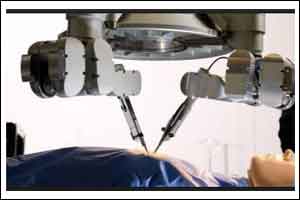
U.S. Food and Drug Administration cleared the Senhance System, a new robotically-assisted surgical device (RASD) that can help facilitate minimally invasive surgery.
“Minimally invasive surgery helps reduce pain, scarring and recovery time after surgery,” said Binita Ashar, M.D., director of the Division of Surgical Devices in the FDA’s Center for Devices and Radiological Health. “RASD technology is a specialized innovation in minimally invasive surgery designed to enhance the surgeon’s access and visualization within confined operative sites.”
RASD, sometimes referred to as robotic surgery, is one type of computer-assisted surgical system. RASD enables the surgeon to use computer and software technology to control and move surgical instruments through one or more tiny incisions in the patient’s body (laparoscopic surgery) in a variety of surgical procedures or operations. The benefits of RASD technology may include its ability to facilitate minimally invasive surgery and assist with complex tasks in confined areas of the body. The device is not actually a robot because it cannot perform surgery without direct human control.
The design of the Senhance System allows surgeons to sit at a console unit or cockpit that provides a 3-D high-definition view of the surgical field and allows them to control three separate robotic arms remotely. The end of each arm is equipped with surgical instruments that are based on traditional laparoscopic instrument designs. The system also includes unique technological characteristics: force feedback, which helps the surgeon “feel” the stiffness of tissue being grasped by the robotic arm; eye-tracking, which helps control movement of the surgical tools and laparoscopic-type controls similar to traditional surgical equipment.
The Senhance System is intended to assist in the accurate control of laparoscopic instruments for visualization and endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrocautery, suturing, mobilization and retraction in laparoscopic colorectal surgery and laparoscopic gynecological surgery. The system is for use on adult patients by trained physicians in an operating room environment.
The manufacturer conducted a clinical study of 150 patients undergoing various gynecological operations with the Senhance System. Clinical outcomes were compared to those described in eight peer-reviewed research publications involving more than 8,000 gynecological operations performed in real-world settings (real-world evidence) using another RASD. In addition, the manufacturer submitted Senhance System operative results involving 45 patients undergoing colorectal procedures in a real-world setting and compared the results to those from peer-reviewed research publications describing the real-world device experience. The FDA concluded that these study data, supported by real-world evidence, along with performance testing under simulated use and worst-case scenario conditions, demonstrated the substantial equivalence of the Senhance System to the da Vinci Si IS3000 device for gynecological and colorectal procedures.
The Senhance System was reviewed through the premarket clearance (510(k)) pathway. A 510(k) notification is a premarket submission made by device manufacturers to the FDA to demonstrate that the new device is substantially equivalent to a legally marketed predicate device.
The FDA granted clearance of the Senhance System to TransEnterix Surgical Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd