- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
New drug protects heart from cardiac rupture after myocardial infarction
There are currently many kinds of drugs for heart failure. Among them, the new drug LCZ696 is recommended by US guidelines as a first-line treatment for chronic heart failure. LCZ696 is better than conventional drugs at reducing cardiac death and hospitalization due to heart failure. Now, researchers from Kumamoto University in Japan have revealed that LCZ696 can prevent cardiac rupture and heart failure following acute myocardial infarction which is one of the causes of chronic heart failure.
Heart disease is the leading cause of death in the world, and the majority of those deaths are caused by heart failure and acute myocardial infarction. In the past, angiotensin converting enzyme inhibitors (ACE inhibitors) or Angiotensin II Receptor Blockers (ARBs) were used in combination with other drugs as the initial therapy for heart failure and acute myocardial infarction. ACE inhibitors and ARBs both inhibit the system responsible for regulating blood pressure, the Renin Angiotensin Aldosterone System (RAAS), which causes high blood pressure when it becomes overactive.
LCZ696 is a novel drug that combines Valsartan, a traditional antihypertensive drug, and Sacubitril, which has an organ-protective effect. Sacubitril inhibits neprilysin which decomposes hormones secreted mainly from the heart called Atrial Natriuretic Peptide (ANP), and B-type Natriuretic Peptide (BNP). A 2014 large-scale clinical trial of patients who had chronic heart failure with reduced ejection fraction (the percentage of blood exiting the heart at each contraction) reported that the number of cardiac deaths and re-hospitalizations due to heart failure was reduced significantly with LCZ696 treatment than with existing ACE inhibitors. LCZ696 is now widely used in Europe and the United States as the first treatment for chronic heart failure.
LCZ696 was established as a chronic heart failure treatment because it suppresses RAAS action and enhances the function of the natriuretic peptide system, which protects the heart. However, it is not yet clear whether it is useful in the acute phase of acute myocardial infarction, which causes chronic heart failure. In a myocardial infarction, the wall between the necrotic and non-necrotic areas of the heart is torn causing rupture and bleeding at the boundary. Ten percent of deaths from myocardial infarction is attributed to cardiac rupture. Therefore, researchers examined the effect of LCZ696, using an artificial myocardial infarction mouse model (a wild-type mouse) .
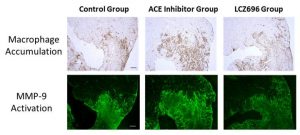
The day after the myocardial infarction model mice were created, they were divided into groups that (1) did not receive any treatment (control group), (2) received an ACE inhibitor, and (3) received LCZ696. The researchers found that the mortality rate due to cardiac rupture occurring within one week after myocardial infarction was significantly lower in the LCZ696 treatment group than in the other two groups. Gene expression in the area of myocardial infarction was analyzed to investigate the mechanism that LCZ696 could inhibit cardiac rupture. The researchers found that the expression of genes involved in inflammation-related cytokines (IL-1β, IL-6) and in tissue degrading MMP-9 was clearly reduced in the LCZ696 treatment group. An increase in the function of the MMP-9 gene is known to be related to the cause of cardiac rupture.
In addition, microscopic observations of the pathological tissue of the heart showed that the number of inflammatory cells was unchanged in all groups, but the activity of MMP-9 was clearly lowered in the LCZ696 treatment group. Furthermore, in experiments using cultured mouse macrophages, researchers confirmed that the inflammation action of cytokines and MMP-9 can be more suppressed using LCZ696 rather than using valsartan or sacubitril by themselves.
"LCZ696 is a new therapeutic drug that improves the prognosis of patients with chronic heart failure," said Dr. Masanobu Ishii of Kumamoto University. "We know that ischemic heart disease, especially myocardial infarction, is one of the causes of chronic heart failure and our study revealed that LCZ696 has a cardio-protective effect against myocardial infarction."
"This improves a patient's prognosis by suppressing cardiac rupture," added Associate Professor Koichi Kaikita. "In the future, it is expected that LCZ696 will be used not only to treat chronic heart failure but also as therapy after acute myocardial infarction."

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd