- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
New drug compound could treat polycystic kidney disease
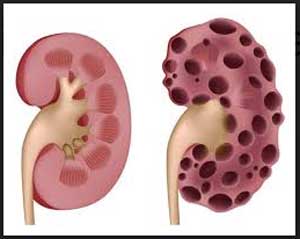
Scientists from the University of Sheffield are part of an international collaboration to develop a new class of drugs to treat common genetic kidney disease- autosomal dominant polycystic kidney disease. Polycystic kidney disease is a major cause of kidney failure.
Working with Glasgow-based biotech company Mironid and colleagues in the US, the Sheffield researchers have carried out successful laboratory tests of a compound developed by Mironid to treat autosomal dominant polycystic kidney disease (ADPKD), a hereditary, progressive disease which affects over 60,000 people in the UK and around 12 million people worldwide.
ADPKD is caused by a genetic fault that disrupts the normal development of kidney cells and causes cysts to form inside the kidneys. The cysts, which are like fluid-filled blisters, grow and eventually destroy normal kidney tissue causing the kidneys to stop working properly. This leaves patients needing life-saving treatments, such as dialysis or a kidney transplant.
In ADPKD cells, cysts develop and grow because they express higher than normal levels of a 'messenger molecule' called cyclic AMP (or cAMP). One of the many known functions of cAMP is to tell cells when to divide and when to secrete fluid.
The new compound discovered by Mironid activates an enzyme called PDE4, whose natural role is to break down cAMP. Researchers at Mironid Ltd describe how the novel small molecule activators of PDE4 enzymes act to supress disease driving cAMP signalling.
Using cell lines and patient tissues derived from ADPKD patient kidneys, researchers at Sheffield and in the US confirmed that when the Mironid compound switched PDE4 on, levels of cAMP in the disease cells were reduced and the number and growth of the cysts were suppressed. The findings are published today (18 June 2019) in the US journal Proceedings of the National Academy of Sciences (PNAS).
Moreover, the reversible nature of the compound means that PDE4 activity may be rapidly and accurately controlled in patients, according to the dose decided by the clinician. This suggests that a future treatment for ADPKD could be tailored to an individual patients' needs and the severity of their disease without the side effects seen with other approaches. In theory, the compound could also suppress cyst formation due to ADPKD in other organs apart from the kidney, especially in the liver.
Professor Albert Ong, a consultant nephrologist and professor of renal medicine who led the experiments conducted at the University of Sheffield said: "Drug development usually focuses on looking for ways to block molecular and chemical processes, not switch them on, so this is a rare mode of action. As a researcher, it's very exciting to be involved in the early stage development of a completely new class of drugs.
"It's also very heartening for me as a clinician since, like most genetic diseases, ADPKD cannot be cured. This discovery gives me hope for the many patients I see in my clinics that there could be another effective new treatment in the future to keep the disease in check for much longer, either on its own or in combination with other drugs such as tolvaptan.
"I would love to think that ADPKD could one day be a disease that people can successfully manage throughout their natural lives, rather than one that could shorten their lives."
Mironid's next step is to refine the compounds in anticipation of being able to develop a new medicine to treat ADPKD patients.
Dr David Henderson, Senior Investigator at Mironid added: "This exciting publication highlights the innovative science behind our LoAc® PDE4 activator technology. These first-in-class small molecules have the potential to address the unmet clinical need in Autosomal Dominant Polycystic Kidney Disease (ADPKD), and potentially have further utility across different therapeutic areas where unb--alanced cellular signalling drives disease progression. Looking to the future, we are excited and enthusiastic in our continued collaboration to translate this novel approach into real therapeutic benefit."
Kidney Research UK has funded work on ADPKD at the University of Sheffield for several years, helping to develop vital knowledge on the mechanism of the disease and ways in which it might be managed.
Chief executive of the charity, Sandra Currie, said: "We are proud to have supported several ADPKD studies led by Professor Ong and his team at Sheffield over the years. Most recently, we funded Professor Ong's investigations into how to control the 'messenger molecule', cyclic AMP, which affects the rate at which cysts grow and expand in the kidneys.
"It is really encouraging news that this collaboration with Mironid has now successfully laboratory-tested a compound which reduces levels of the cyclic AMP molecule. This could bring new hope to the 12 million people living with ADPKD worldwide -- we look forward to further developments."
For more details click on the link: DOI: 10.1073/pnas.1822113116

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd