- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Management of Graves’ Disease : 2018 European Thyroid Association Guideline
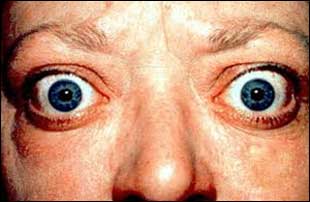
The 2018 European Thyroid Association Guideline for the Management of Graves’ Disease was published in European Thyroid Journal. The target audience for this guideline are physicians providing care for patients with Grave's Disease. In this document, rational medical practice is outlined. This guideline does not replace clinical judgment, individual decision making, or the wishes of the patient or family. Rather, each recommendation should be evaluated in light of these elements in order that optimal patient care is delivered.
Key Recommendations:
1 The measurement of TSH-R-Ab is a sensitive and specific tool for rapid and accurate diagnosis and differential diagnosis of Graves’ hyperthyroidism.
2 When technically available, differentiation of TSH-R-Ab functionality is helpful and predictive in Graves’ patients during pregnancy/postpartum, as well as for extrathyroidal manifestations.
3 US examination, comprising conventional grey scale analysis and color-flow or power Doppler examination is recommended as the imaging procedure to support the diagnosis of Graves’ hyperthyroidism.
4 Scintigraphy of the thyroid is suggested when thyroid nodularity coexists with hyperthyroidism, and prior to RAI therapy.
5 Patients with newly diagnosed Graves’ hyperthyroidism should be treated with ATD. RAI therapy or thyroidectomy may be considered in patients who prefer this approach. 1, ∅∅∅∅
6 MMI (CBZ) should be used in every non-pregnant patient who chooses ATD therapy for Graves’ hyperthyroidism.
7 MMI is administered for 12–18 months then discontinued if the TSH and TSH-R-Ab levels are normal.
8 Measurement of TSH-R-Ab levels prior to stopping ATD therapy is recommended, as it aids in predicting which patients can be weaned from the medication, with normal levels indicating a greater chance of remission.
9 Patients with persistently high TSH-R-Ab at 12–18 months can continue MMI therapy, repeating the TSH-R-Ab measurement after an additional 12 months, or opt for RAI or thyroidectomy.
10 Patients should be informed of the potential side effects of ATD and the necessity of informing the physician promptly if they should develop jaundice, light-colored stools, dark urine, fever, pharyngitis, or cystitis.
11 In patients taking ATD, a differential white blood cell count should be obtained during febrile illness and/or pharyngitis, and liver function should be assessed in those who experience jaundice, light-colored stools, or dark urine.
12 The beta-adrenergic blockade is recommended in all suitable patients with Graves’ hyperthyroidism.
13 If a patient with GD becomes hyperthyroid after completing the first course of ATD, definitive treatment with RAI or thyroidectomy is recommended. Continued long-term low-dose MMI can be considered in patients, not in remission who prefer this approach.
14 Treatment of SH is recommended in Graves’ patients > 65 years with serum TSH levels that are persistently < 0.1 mIU/L.
15 ATD should be the first choice of treatment of Graves’ SH.
16.A multimodality treatment approach to GD patients with thyroid storm should be used, including ATD therapy, glucocorticoid administration, beta-adrenergic blockade, cooling blankets, volume resuscitation, nutritional support, respiratory care, and monitoring in an intensive care unit.
17 There are no absolute indications for RAI therapy, but it is often recommended for patients with side-effects to or recurrence after a course of ATD. 1, ∅∅○○
18 Verbal as well as written information on all aspects of efficacy and potential side-effects of RAI therapy should be provided. 1, ∅∅○○
19 If ATD are used before RAI therapy they should be paused around 1 week before and after therapy in order not to decrease the efficacy of RAI therapy. 1, ∅∅∅∅
20 No dose calculation can secure long-term euthyroidism and it is fully acceptable to offer a fixed dose of RAI.
21 Pregnancy and breastfeeding constitute absolute contraindications to RAI therapy. 1, ∅∅∅○
22 Conception should be postponed until at least 6 months after RAI in both males and females.
23 If used in children, ablative doses aiming at rapid hypothyroidism should be administered.
24 If surgery is selected, total thyroidectomy is the procedure of choice, and should be performed by a skilled surgeon with high annual volumes of thyroidectomies.
25 Euthyroidism should be restored by ATD prior to surgery to avoid peri- or postoperative exacerbation of thyrotoxicosis.
26 Vitamin D deficiency should be corrected to reduce the postoperative risk of hypocalcemia.
27 A solution containing potassium iodide can be given for 10 days prior to surgery.
28 In patients with GO, hyperthyroidism should be promptly controlled by ATD, and euthyroidism stably maintained.
29 Patients treated with RAI should receive steroid prophylaxis if mild and active GO preexists or there are risk factors for RAI-associated GO occurrence or progression.
30 In patients with moderate-to-severe and active GO, treatment of GO should be the priority. Euthyroidism should be promptly restored with ATD and stably maintained.
31 Patients with sight-threatening GO should be treated with ATD. 1, ∅∅○○
32 Treatment for hyperthyroidism in patients with inactive GO can be selected independently of GO.
Read Also:LT4 and LT3 combo effectively improves QOL in a subset of hypothyroid patients
33 Women with GD of reproductive age should be offered preconception counseling and be stably euthyroid before attempting pregnancy.
34 Women with GD should be instructed to immediately confirm pregnancy and contact their physician.
35 Women treated with MMI should be switched to PTU when planning pregnancy and/or during the first trimester of pregnancy.
36 All patients with a history of autoimmune thyroid disease should have their TSH-R-Ab serum levels measured at the first presentation of pregnancy using either a sensitive binding or a functional cell-based bioassay and, if they are elevated, again at 18–22 weeks of gestation. 1, ∅∅∅∅
37 If the maternal TSH-R-Ab concentration remains high (> 3 times the cut-off), monitoring of the fetus for thyroid dysfunction throughout pregnancy is recommended.
38 During pregnancy the lowest possible dose of ATD should be given and the block-and-replace ATD regimen is discouraged.
39 Maternal FT4 (TT4) and TSH should be measured every 2 weeks after the initiation of therapy, and every 4 weeks after achieving the target value.
40 A change from PTU to MMI should be considered if ATD are required after 16 weeks gestation.
41 In women on a low dose of MMI (<5–10 mg/day) or PTU (<50–100 mg/day), ATD may be stopped during gestation prior to weeks 6–10.
42 Lactating women with GD should be offered the same treatments as non-lactating women.
43 MMI is recommended during lactation, given the concerns about PTU-mediated hepatotoxicity.
44 Older patients who have had atrial fibrillation, cardiac failure or cardiac ischemic symptoms precipitated by hyperthyroidism should undergo definitive therapy, usually RAI.
45 Long-term MMI (CBZ) should be considered as a satisfactory treatment for older individuals with mild GD.
46 PTU should be avoided in children and adolescents.
47 Long-term MMI (CBZ) should be the mainstay of treatment in children with GD.
48 Thyroidectomy is the primary definitive therapy in childhood, but in postpubertal children, RAI can be considered.
49 Graves’ hyperthyroidism precipitated by an immunomodulatory therapy is not a mandatory indication to stop that precipitating treatment, nor is it a mandatory indication for definitive therapy for hyperthyroidism.
50 Sequential monitoring of serum TSH-R-Ab levels can be used to guide the duration of ATD therapy in patients with immune reconstitution GD.
GD is an organ-specific autoimmune disease whose major manifestations are owing to circulating autoantibodies (Ab) that stimulate the thyroid-stimulating hormone receptor (TSH-R) leading to hyperthyroidism and goiter.
For reference log on to https://doi.org/10.1159/000490384

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd