- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
MACI implant regrows Knee Cartilage - Good option for knee transplant
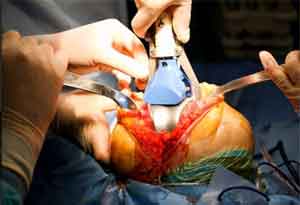
Vanderbilt’s Scott Arthur, M.D., recently performed the state’s first knee surgery using a newly approved implant containing a patient’s regrown cartilage cells.
Trademarked under the name MACI, the implant was approved by the U.S. Food and Drug Administration in December. It is the first FDA-approved product that regrows knee cartilage cells on scaffolds. Previously, this type of regenerative therapy, which entails extracting healthy cartilage cells from a patient’s knee then re-growing them in a laboratory, was available only in liquid form.
“In the first generation, called Carticel, you would have to take a patch and sew it into the cartilage and then inject the liquid containing the cartilage cells behind the patch,” said Arthur, assistant professor of Clinical Orthopaedic Surgery and Rehabilitation. “With MACI, the M stands for matrix, so it is a collagen matrix and the patient’s own cartilage cells are implanted into the matrix. In the operating room, we shape the patch to fit into the cartilage defect.”
MACI (Matrix-induced autologous chondrocyte implantation), a Vericel Corp. product, is approved for repairing cartilage defects in the knee for patients ages 18-54. The scaffolding better secures the regrown cartilage.
“It’s a good option for some joints with really difficult problems,” Arthur said. “A lot of patients who would benefit from MACI tend to be younger patients, athletic patients who want to regain a high level of function.”
The MACI implant is approved for the repair of full-thickness cartilage defects of the knee, which is a fairly select patient group. Similar innovations with wider applications are on the horizon.
“This is the next frontier in orthopaedics — trying to figure out ways to use your body’s own ability to repair itself,” Arthur said. “The future is utilizing stem cells and growth factors and cell therapy to encourage and enhance healing and recovery. That’s where we are going. We are in the infancy of trying to figure a lot of this out.”

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd