- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Use of Laser Dermatological Method to Remove Tattoos
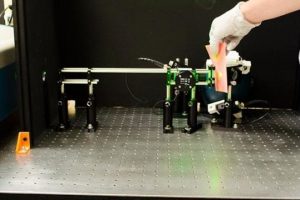
Laser techniques come with risks, including eye damage. Open-air transmission, in which the clinician holds the laser at a distance from the patient, is typical during normal dermatological procedures and presents a hazard to both the patients' and doctors' eyes. Paul J.D. White side, a doctoral candidate in the MU Division of Food Systems and Bio engineering, devised a system that will not only improve the process, but will be safer for both clinicians and patients.
"The system we developed uses ultrasonic pulsation in conjunction with a clinical laser to alter the properties of skin tissues during the procedure," White side said. "We've named the technique 'sonoillumination,' and we're hopeful that the procedure will be available widely in the near future."
Whiteside and his team, including adviser, Heather K. Hunt, an assistant professor of bioengineering in the MU College of Engineering, tested the sonoillumination system on porcine skin tissue samples. Using various amplitudes and pulses, the instruments they developed were tested on the samples and showed great promise for the clinical setting. Whiteside presented his technique to clinicians on April 9, 2017, at the annual conference of American Society for Laser Medicine and Surgery (ASLMS).
"Pork skin samples are very close to human skin samples, so the initial results we saw are promising for human applications," Hunt said. "'Sonoillumination' will be extremely beneficial for clinicians and the ASLMS presentation allowed us to demonstrate the system to the people who actually will be using the technology once it's commercialized."
Nicholas Golda, associate professor of dermatology and director of dermatology surgery at the MU School of Medicine, echoed the merits of the sonoillumination system and the effect it will have on dermatology.
"Our goal is to provide patients with safer, more effective treatment options that potentially lower the number of treatments needed," Golda said. "This new technology may also provide physicians with a safer, more controllable option for treating patients."
The team co-authored the paper, "Ultrasonic modulation of tissue optical properties in ex vivo porcine skin to improve transmitted trans dermal laser intensity," which recently was accepted for publication by the society's journal, Lasers in Surgery and Medicine. The sonoillumination research was funded in part by a 2015 Fast Track grant from the University of Missouri System.
The team is in the planning stages of developing a start-up company to commercialize the technique. Products produced by White side and the team highlight the University's impact on the state's economic development efforts, including commercialization of research conducted at Mizzou, workforce development and job growth, quality of life improvements for residents, and attracting corporations and businesses to the state. Over the last five years, companies commercializing MU technologies have secured hundreds of millions of dollars in investments and grants to advance their commercialization efforts. In 2016, the Office of Technology Management and Industry Relations reported that Mizzou received $14.9 million in revenue from more than 40 technology licenses.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd