- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
IV Tranexamic Acid a primary treatment for PPH- WHO Guidelines
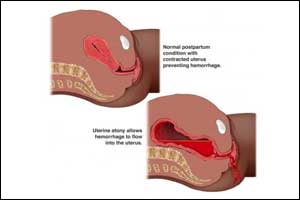
The World Health Organization (WHO) has published an updated recommendation on the use of tranexamic acid(TXA) for PPH treatment, based on the evidence generated by the WOMAN trial, which supersedes the 2012 recommendations. The WHO Guideline Development Group (GDG) was convened in August 2017 to review and consider the updated analysis on tranexamic acid, and an official recommendation was made by the GDG to include the use of tranexamic acid as a first line treatment for all causes of PPH.
According to WHO, “clinically diagnosed postpartum hemorrhage” refers to a clinically estimated blood loss of more than 500 mL after vaginal birth or 1000 mL after cesarean section or any blood loss that is sufficient to compromise hemodynamic stability. The majority of these deaths or near miss events occur within twenty-four hours after birth; most are preventable by timely intervention using standard treatment protocols, including uterotonics and surgical interventions.
The World Health Organization (WHO) recommends that intravenous tranexamic acid (TXA) be considered as part of the standard PPH treatment package. It should be administered as soon as possible after onset of bleeding and within 3 hours of birth, in addition to standard care for women with clinically diagnosed postpartum hemorrhage (PPH). However, TXA for PPH treatment should not be initiated more than 3 hours after birth, as it offers no advantages. The fixed dose of TXA is 1 g in 10 mL (100 mg/mL) IV at 1 mL per minute (i.e. administered over 10 minutes), with a similar second dose of 1 g IV if bleeding continues after 30 minutes. It should be given only through the IV route for treatment of PPH. Research on other routes of TXA administration is a priority, especially in low resource settings where personnel trained in IV administration may not be available.
The World Maternal Antifibrinolytic (WOMAN) Trial was a randomised, double-blind, placebo-controlled trial, that recruited women aged 16 years and older with a clinical diagnosis of PPH (regardless of mode of delivery). This 21 country study was conducted in nearly 200 hospitals, and recruited over 20, 000 women. Women with clinically evident PPH were randomized to receive either 1 g intravenous tranexamic acid or matching placebo, in addition to usual care. If bleeding continued after 30 minutes, or stopped and restarted within 24 hours of the first dose, a second dose was used. The primary endpoint was a composite of death from all-causes or hysterectomy within 42 days of giving birth. The composite primary endpoint was not reduced with tranexamic acid (5·3% vs 5·5%, RR 0·97, 95% CI 0·87-1·09; p=0·65). However, death due to bleeding was significantly reduced in women given tranexamic acid (1·5% vs 1·9%, RR 0·81, 95% CI 0·65–1·00; p=0·045), especially in women treated within 3 hours of giving birth (1·2% vs 1·7%, RR 0·69, 95% CI 0·52–0·91; p=0·008). However, all other causes of death did not differ significantly by group. Hysterectomy was not reduced with tranexamic acid (3·6% vs 3·5%, RR 1·02, 95% CI 0·88–1·07; p=0·84).
The trial authors concluded that tranexamic acid reduces death due to bleeding in women with clinically diagnosed PPH and that early treatment appears to optimize benefit.
The GDG did a meta-analysis of the data and of the effects of timing of administration, which indicated that delay in treatment appears to reduce benefit, decreasing by 10% for every 15 min delay, and with no benefit seen after 3 h. Giving TXA beyond 3 h after birth is suspected to be potentially harmful. Importantly, giving the drug earlier after birth appears to increase benefit; hence it should be given as soon as possible for treatment of post-partum hemorrhage.
Treatment with tranexamic acid should be avoided in women with a clear contraindication to antifibrinolytic therapy (e.g., a known thromboembolic event during pregnancy). The 2017 recommendation applies to giving the drug intravenously only, since the benefits and potential harms of other routes of administration are not yet known and are a research priority.
A maternal death has devastating effects on the family and particularly her offspring. Safe, effective and affordable PPH treatments are critical to saving the lives of pregnant women globally, and the findings of this trial have important implications for the delivery of high-quality maternity care and preventing maternal deaths. The drug is easily available and already in wide use in surgery; this study widens the scope of use in PPH with significant improvement in prevention of maternal deaths due to bleeding alone. It is a useful addition to the existing armamentarium of drugs used in the treatment of PPH.
Ref:
1.Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum hemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. The Lancet Volume 389, No. 10084, p2105–2116, 27 May 2017
http://www.thelancet.com/journals/langlo/article/PIIS2214-109X(17)30428-X/fulltext
WHO recommendation on tranexamic acid for the treatment of postpartum hemorrhage
http://www.who.int/reproductivehealth/publications/tranexamic-acid-pph-treatment/en/
Dr. Sunanda Gupta
The author MBBS & MD, MPH (Obstetrics & Gynaecology) and is Head of Department, Obs/Gyn, World College of Medical Sciences and Research, Haryana. She is a member Editorial Board, Obstetrics & Gynaecology at Specialty Medical Dialogues.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd