- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
ICMR: Frequently Asked Questions on Stem Cells & their Clinical Application
Basics of Stem Cells
- What is a cell?
- A cell is the building block of our body. It is the smallest structural and functional unit of the body.
- There are different types of specialized cells that perform different functions.
- Humans have an estimated 100,000,000,000,000 (one hundred trillion) cells and more than 200 different types of cells (liver cells, skin cells, muscle cells, etc.).
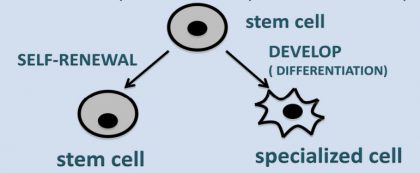
2. What is stem cell?
A stem cell is a naïve/immature/unspecialized cell that can
i. divide to form similar cells (self renewal) and
ii. develop into different specialized cells that perform distinct function

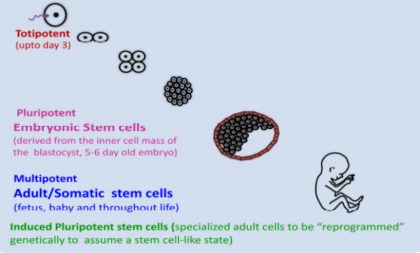
3. Are all the stem cells same? What are the different types of stem cells?
No, all the stem cells are not same. There are many different types of stem cells that come from different places in the body or are formed at different times in our lives. These include
• Embryonic stem cells (ESCs) which exist only at the earliest stages of development
Adult stem cells also called somatic stem cells that appear during fetal development and are present in different tissues of our bodies throughout life.
• Induced pluripotent stem cells or iPSCs cells are not found in the body but engineered in laboratory from cells of the body, such as those from the skin. The iPSC cells have properties similar to those of embryonic stem cells.
Depending on their ability (potency) to develop into different specialized cells, stem cells can be
• Totipotent stem cells- These cells can form all cells of the body including the ones in the extra embryonic membranes (placenta). In humans, the 2 cell staged zygote is totipotent.
• Pluripotent stem cells – These stem cells can become virtually any cell in the body except those needed to support and develop a fetus in the womb. ESCs and iPSCs are pluripotent stem cells.
• Multipotent stem cells can give rise only to a small number of different cell types
4. What are embryonic stem cells (ESCs)? What is the source of embryos to create ESCs?
- ESCs are pluripotent stem cells that can renew themselves and become virtually any cell in the body except those needed to support and develop a fetus in the womb.
- Eggs fertilized by sperm begin to divide into multiple cells, but do not begin to form organs and tissues for at least two weeks. During this early developmental period, the cells that will ultimately give rise to the developing fetus can be encouraged to grow indefinitely in the laboratory as stem cells that are not committed to any particular tissue.
- Human ESCs are derived from the embryos that are 5-6 days old. At this stage, the embryo is a ball of approximately 100 cells called a blastocyst and is no bigger than a grain of sand. Human embryonic stem cells were isolated in 1998.
Embryos used to generate human ESCs come from several sources:
• ESCs can be derived from donated embryos left after in-vitro fertilization (IVF).
• IVF embryos analyzed by preimplantation genetic diagnosis can also be used to generate ESCs. An alteration of this technique allows generation of ESCs from single cells removed from embryos in a process similar to preimplantation genetic testing.
• ESCs can be derived from eggs that have been parthenogenetically activated; that is, the eggs are induced to divide without being fertilized by sperm. This new category has received less public debate because it is less common and less well understood.
• Somatic cell nuclear transfer (SCNT) can be used to produce embryos from somatic or adult cells using donated enucleated eggs, and then ESCs can be generated from the resulting embryos.
5. What are adult stem cells?
Adult stem cells or somatic stem cells or tissue-specific stem cells- are the reserve supply of cells that can multiply when needed for repair of adult organs and tissues.
• These cells are present in our body in almost all the organs.
• They are multipotent i.e. they can give rise to a limited number of mature cell types, usually corresponding to the tissues in which they reside. Most common example is the blood forming (hematopoietic) stem cells from bone marrow that give rise to the different blood cells in our body.
• Some tissue-specific stem cells can only give rise to one or two mature cell types and are called unipotent and bipotent, respectively. Stem cells found in the skin produce new skin cells, and are example of unipotent stem cells.
6. What are induced pluripotent stem cells (iPSCs)?
These cells are not found in the body but made in laboratory from cells of the body, such as those from the skin. The expression certain genes (genes are one of the major components that control the fate of the cell) of adult body cells with specialized function is manipulated to reprogramme the somatic cells back to a pluripotent state.
• The iPSC cells have properties similar to those of embryonic stem cells.
• Human iPSCs were generated in 2007.
Stem Cell Research in India
1. Is there stem cell research activities going on India? In what way Government of India supported these activities?
Yes.
The Government of India has been supporting basic as well as clinical research through national funding agencies like the Indian Council of Medical Research (ICMR), Department of Biotechnology (DBT) and Department of Science and Technology (DST). This has resulted in the establishment of state-of-art infrastructure at over 40 premier health research and educational institutes in addition to supporting industry initiatives in India.
2. Are there any guidelines governing stem cell research in India?
Yes, since 2007 guidelines are available to direct scientist and clinicians working in the field of stem cell research. They have to work in compliance with these.
• MoHFW, Government of India is committed towards stem cell treatments that are safe and have proven efficacy. The Guidelines for Stem Cell Research and Therapy in 2007 was a step towards this commitment, which were revised after public consultations/opinion and released as National Guidelines for Stem Cell Research (NGSCR-2013).
• These were further updated by incorporating the recent advances in the field and have also been harmonized with the existing rules and regulations in the country and were released by Ministry of Health & Family Welfare as National Guidelines for Stem Cell Research (NGSCR), 2017 on 12th October 2017.
3. What kind of stem cell research is covered by NGSCR 2017?
Both basic and clinical research involving any kind of human stem cells and their derivatives is to be done in compliance with NGSCR 2017.
• The guidelines do not apply to research using non-human stem cells and their derivatives.
• Further, these do not apply to use of hematopoietic stem cells for treatment of various hematological, immunological and metabolic disorders since these have already been established as a standard of medical care.
• Protein-rich plasma (PRP) and autologous chondrocyte/osteocytes implantation does not fall under the purview of these guidelines as they are categorized as other cell based applications and not stem cell transplantation.
4.Who has to follow the NGSCR 2017?
All the stakeholders including individual researchers, organizations, sponsors, oversight/regulatory committees and all others associated with both basic and clinical research involving any kind of human stem cells and their derivatives.
5. What is the mechanism of monitoring the stem cell research activities in the country?
Two levels of monitoring mechanism have been established: one at the national level focusing primarily on policy and the other, a more self-regulatory system of review at the institutional level.
• The National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT) has been constituted and notified by Department of Health Research (DHR), Ministry of Health and Family Welfare, Govt. of India as an independent body of experts representing diverse areas of biomedical research, concerned government agencies and other stakeholders.
• The Institutional Committee of Stem Cell Research (IC-SCR), on the other hand, operates at the institutional level with members having specific expertise as per these guidelines. It is mandatory for them to register with NAC-SCRT and submit annual report on their scientific activities for effective functioning.
• All Clinical trial need approval of Cell Biology Based Therapeutic Drugs Evaluation Committee (CBBTDEC) of Central Drug Standards Control Organization (CDSCO).
Basic Research
1. What is meant by basic research?
Basic fundamental research is an essential component of biomedical science, intended to enhance knowledge and understanding of a subject without necessarily leading to immediate practical solutions and/or therapeutic application.
2. What is the importance of basic research in field of stem cell and how it has been useful so far?
A focus on basic aspects of research in stem cell biology is important to advance our understanding on the mechanisms responsible for stemness, role of niche, dormancy, recruitment, plasticity and their ability to repair and regenerate etc.
3. What is included in the basic research?
It includes establishing in vitro cell culture systems to investigate stem cells and progenitors of different lineages and understand stages of cell differentiation. In vivo studies i.e. stem cell research in animals is also a part of basic research.
4. How basic research been useful so far?
Research on human ESCs has led to new knowledge about embryo development. Breakthrough in iPSC technology has revolutionized the field of stem cell biology and has led to the generation of human disease specific models to understand the underlying pathophysiology. In vitro stem cell cultures have found application in drug discovery and toxicity screening.
5. In what way NGSCR 2017 applies to basic stem cell research?*
The basic stem cell research largely falls under the permissible category that can be conducted with prior approval of IC-SCR and Institutional Ethics Committee (IEC) or Institutional Animal Ethics Committee (IAEC) and includes
• In vitro studies using stem cells and/or established stem cell lines
• Establishment of new human ESC lines from spare embryos or iPSC lines from fetal/adult tissues or somatic stem cell lines from fetal or adult tissues.
• In vivo studies in experimental animals (other than primates), with established cell lines from any type of human pluripotent stem cells viz. ESCs, iPSCs, including their differentiated cells, and human SSCs (fetal, neonatal or adult) from any tissue, are permitted with the prior approval of IC-SCR and IAEC.
Basic research studies falling under restrictive area of research require additional arm of oversight/monitoring (such as IAEC/CPSCEA/ISBC/RCGM/NAC-SCRT approvals, as applicable) due to contentious issues involved. It includes:
• Creation of human pre-implantation embryos by In vitro fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), Somatic Cell Nuclear Transfer (SCNT) or any other method with the specific aim of deriving ESC lines for any purpose.
• Research involving introduction of human ESC/iPSC/SSCs into animals (including primates), at embryonic or fetal stages of development for studies designed to understand the patterns of differentiation and integration of human cells into non-human animal tissues.
• Studies on chimeras where stem cells from two or more species are mixed together at any stage of early development (embryonic or fetal), for understanding patterns of development and differentiation.
• Genome modification including gene editing (for example by CRISPR-Cas9 technology) of stem cells, germ-line stem cells or gamete and human embryos is restricted only to in vitro studies.
The following areas of fall under the prohibited area of research and cannot be done pursued under
any circumstances:
• Research related to human germ line gene therapy and reproductive cloning.
• In vitro culture of intact human embryos, regardless of the method of their derivation, beyond 14 days of fertilization or formation of primitive streak, whichever is earlier.
• Research involving implantation of human embryos (generated by any means) after in vitro manipulation, at any stage of development, into uterus in humans or primates.
• Breeding of animals in which any type of human stem cells have been introduced at any stage of development, and are likely to contribute to chimeric gonadal cells.
Clinical Research
1. Do stem cells fall under the category of ‘drug’?
- Stem cells and their derivatives fall under definition of ‘Drug’ as per the Drugs and Cosmetics Act 1940, and are categorized as ‘Investigational New Drug (IND)’ or ‘Investigational New Entity (INE)’ when used for clinical application.
- Hence clinical trials are a must before these can be applied for any unproven applications and the principles of bioethics and regulation must be followed accordingly before initiating clinical trials.
2. What is Clinical research/study/ trial?
- Clinical research/study/trial test capability of treatments in human volunteers or patients to see whether they should be further investigated or commercially used in general population.
- A treatment could be a drug (medicine), medical device, or a stem cells, vaccine, blood product, or gene therapy.
It must be noted that participants of a clinical trials can’t be charged for the trial treatment they are getting.
3.Why clinical trials are important?
- Often, a promising lead in the lab does not translate into a safe and effective treatment for humans.
- There may be several risks and side effects associated with the administration of new drug/product/process in humans. Thus any new drug/products/process requires a thorough evaluation in the form of a well designed clinical trial.
- Any ill effects that can be caused by a new drug/products/process can only be known when the patients are followed up for appropriate period which can only be done in a clinical trial.
4. Have there been stem cell clinical trials in India? What have been the results?
Yes, different Government agencies like ICMR, DBT, DST etc have funded and are funding stem cell related clinical trials.
• The experience of Indian funding agencies so far has shown that stem cell therapy is yet not an effective option in several conditions.
• The pilot studies supported in spinal cord injury and age related macular disorder did not show any benefits of stem cell treatment.
• Similarly, multicentric trials in chronic stroke and myocardial infarction did not show statistically significant improvement.
• However, exceptional results/positive trends have been obtained in critical limb ischemia caused by Burger’s disease and that has been granted conditional approval by CDSCO to substantiate the outcome by doing more cases.
5. Which agency regulates clinical trials in India?
All clinical trials are regulated by CDSCO and require prior approval of DCG(I) before they can be started.
6. Is there a difference between clinical trial and clinical practice?
Yes.
• In clinical practice specialised doctors uses established treatments.
• In clinical trial proof/evidences are collected in scientific and ethical manner to establish a treatment.
7. Which institutions/hospitals can conduct clinical trials?
As per NGSCR 2017, only those entities that fulfill the following requirements defined in NGSCR, 2017, can conduct clinical trial with stem cell:
a. Clinical trials can be permitted only in institutions/hospitals having registered IC-SCR (with NACSCRT) and IEC (with CDSCO). For multi-centric clinical trials, all participating sites should obtain approvals form their own IC-SCR and IEC.
b. These can only be conducted in a medical institution/hospital with adequate infrastructure and clinical facilities in accordance with Para 2 (1)(ii) of Schedule Y, Drugs and Cosmetic Act 1940 and Rules 1945.
8. What are the requirements for stem cell clinical trials?
a. Clinical trials should be in compliance with Schedule Y of Drugs and Cosmetics Act and GCP Guidelines of CDSCO (www.cdsco.nic.in) as well as ICMR-Ethical Guidelines for Biomedical and Health Research involving Human participants 2017 http://www.icmr.nic.in/ethical_guidelines.pdf
b. All clinical trials on stem cells shall be registered with Clinical Trial Registry India (CTRI). http://ctri.nic.in/Clinicaltrials/login.php
c. The cells or cell-based products used in the trial should be processed in a CDSCO certified GLP and GMP facility (Schedule L1 and M of Drugs and Cosmetic Act, 1940 and Drugs and Cosmetics Rules, 1945.
d. Clinical trial must have a medical specialist registered with MCI and holding MCI approved post graduate qualification in the subject domain of the trial.
e. All medical professionals involved in clinical trials should have a valid GCP certification.
9. Who can participate in a clinical trial?
Any patient/ healthy person who match the requirement of the given trial can participate.
10. Can a patient be charged if he is participating in a clinical trial? There are several clinics/hospitals that claim to be doing clinical trials, but ask for money, is that OK.
No, participants enrolled for clinical trials are liable to pay any charges towards procedures, investigations and/or hospitalization related to the trial.
11. How to participate in a study?
• ICMR will soon start a portal for such information. Till then, CTRI site can be checked for ongoing trials in the field.
• For international trials, following link listing the international trials registries in different countries can be referred to https://www.hhs.gov/ohrp/international/clinical-trialregistries/index.html
12. From where do the cells used in clinical trials come from?
• The stem cells transplanted into patient can be from his/her own body (known as ‘autologous’ transplant) or
• Non-self source (known as allogeneic transplant) which include fetal, embryonic, cadaver or a living donor. The donor or the tissues first need to be checked for matching before it can be given to the patient.
• It is important that prior video consent of donor is obtained for procurement of biological material for research or allogenic transplant.
Stem Cell Treatment
1. What is the status of Stem Cell treatment in India as well globally?
In India as well as globally, only blood stem cells from bone marrow to treat blood cancers and different blood disorders are permitted.
The clinical use in any other disease or use of any stem cells other than these is still in research stage.
2. At present for which diseases, stem cell therapy is available?
• Though stem cell therapies are being touted as a panacea for all ills, but at this point in time, only the blood forming (hematopoietic) stem cells from bone marrow or umbilical (nadi) cord blood are routinely used to treat blood cancers and different blood disorders. This is known as bone marrow transplant (BMT) or hematopoietic stem cell transplant (HSCT).
• For other diseases, studies are being conducted to find if stem cells can be helpful in curing these diseases.
3. Is stem cell treatment available in India for diseases other than BMT/HSCT for some blood cancers and blood disorders? Are there Government Hospitals where stem cell therapy is being provided?
• No. At present, stem cell therapy other than BMT/HSCT for conditions as listed in the NGSCR 2017 is neither available nor permissible.
• No Government hospital offers stem cell therapy for unproven conditions.
• In case a clinical study is ongoing, patients may avail the treatment as part of the trial if they fit into the requirements of that trial.
4. Can hospitals offer Stem Cell Treatment for different ailments?
No. As per NGSR 2017, it is considered unethical and a malpractice and is violation of the existing National Guidelines for Stem Cell Research, a clinician/hospital claims to commercially offer stem cell therapy as a cure for any disease.
5. There are several clinics that offer stem cell based therapies saying they have done clinical studies/trials and/or provide testimonies of patients who have been cured from the stem cell treatment done at their clinic. How valid are such trials/studies/testimonies?
• It must be kept in mind that best studies/trials are ‘randomized controlled trials’ which are considered level I evidence and policy decisions are mostly based on meta analysis of the level I evidence studies.
• Claims/testimonies/case studies are individual opinions/single case observations that are considered weak evidences as they lack proper design, have bias and do not provide statistically significant results.
6. There are several clinicians/hospitals/entities advertising and offering stem cell therapy for many incurable diseases through electronic and print medium. What actions can be taken against such advertisements?
It may be noted that actions can be taken against the erring clinicians/entities as per the following existing rules and regulations.
a. The advertising and publicity through any mode by clinicians is not permitted as per Chapter 6 of the Indian Medical Council (Professional Conduct, Etiquettes and Ethics) Regulation. It is mandated that the MCI and Medical Councils of respective state should initiate action on the erring clinicians for violation of code of ethics prescribed by it either taking suo moto cognizance or acting on any complaint received by them.
(Available at: https://www.mciindia.org/documents/rulesAndRegulations/Ethics Regulations2002.pdf )
b. The Drugs and Magical Remedies (The Objectionable Advertisements) Act- 1954 –prohibits misleading advertisements relating to drugs and magical remedies. DGHS and relevant state authorities are mandated to take necessary action for violation of this act. (Available at: http://lawmin.nic.in/ld/P-ACT/1954/A1954-21.pdf).
c. The advertisement of treatment of several diseases as listed in Schedule J of Drugs and Cosmetics Act, 1940 and rules therein (Annexure VII) is not permissible. Hence publicity claiming available cure for these conditions using stem cells and its derivatives is prohibited. CDSCO, DGHS and relevant state authorities are mandated to take necessary action for violation of this act.
d. No advertisement which violates the code for self regulation in advertising, as adopted by the Advertising Standards Council of India (ASCI), Mumbai for public exhibition, from time to time, shall be published. (Available at: https://ascionline.org/images/pdf/code_book.pdf). The complaints can be made online at the following web link of ASCI https://ascionline.org/index.php/lodge-ur-complaints.html
7. Which agencies can be approached to report unethical practices of clinicians/hospitals/entities that are offering unproven Stem Cell Treatments?
If you come across any hospital/ doctor/ clinic offering un proven therapy and also charging for the same OR have been charged by any hospital/doctor/clinic for stem cell therapy, you can submit the complain with details to following agencies:
• State Medical Council
• Ethics Committee Medical Council of India
• State Indian Medical Association
• State Office of Central Drug Standards Control Organization
• Indian Council of Medical Research
For more details click on the given link:
http://www.icmr.nic.in/icmrnews/FAQ_Stem_cell.pdf

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd