- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
ICMR Antimicrobial guidelines for prophylaxis and treatment of Surgical Site Infections
Surgical site infections (SSIs) are one of the most common health care associated infections (HCAIs) and represent a substantial cause of morbidity with 2-11-fold higher mortality. Patients developing SSIs are 60% more likely to be admitted to an ICU, are more than five times more likely to be readmitted to the hospital, and are twice as likely to die as similar patients without SSIs. SSIs complicate 3,00,000-5,00,000 surgeries per year in the USA alone, and are believed to result in US$5-10 billion of excess health expenditures, with 7- 10 days of increased length of hospital stay. SSI rates have become a universal measure of quality in hospital-based surgical practice, since they are probably the most preventable type of HCAI.
Peri-operative antimicrobials administered as prophylaxis for SSIs account for the majority of in-hospital antimicrobial prescriptions. Usually, long courses of antibiotic prophylaxis are administered, which are often associated with increasing antimicrobial resistance, super-infection with resistant pathogens, toxicity and unnecessary cost. Rampant and unnecessary administration of antibiotics is one of the major contributors for development of drug resistance.
In a systematic review on antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures, a single dose antibiotic prophylaxis was found to significantly reduce the risk of deep surgical site infections. Several studies have justified the use of short courses of a single cephalosporin for clean surgeries, since these act on the most likely organisms causing SSIs.
Indian Council of Medical Research, Department of Health Research has issued the ICMR Antimicrobial guidelines for prophylaxis and treatment of Surgical Site Infections. Following are the major recommendations :
Case definitions
Surgical Wound Classification
- Class I/Clean: uninfected operative wound in which no inflammation is encountered & respiratory, alimentary, genital, or uninfected urinary tract is not entered. Operative incisional wounds following blunt trauma are included here.
- Class II/ Clean-Contaminated: Operative wound in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination.
- Class III/Contaminated: Open, fresh, accidental wounds. Operations with major breaks in sterile technique or gross spillage from the GIT.
- Class IV/Dirty-Infected: Old traumatic wounds with retained devitalized tissue and those that involve existing clinical infection or perforated viscera.
Table 1 Criteria for Defining a Surgical Site Infection (SSI)CDC ; ref 1 |
Superficial Incisional SSIInfection occurs within 30 days after the operation and infection involves only skin or subcutaneous tissue of the incision and at least one of the following:
Note: Specific criteria are used for identifying infected episiotomy and circumcision sites and burn wounds. Deep incisional SSIInfection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and infection involves deep soft tissues (e.g., fascial and muscle layers) of the incision and at least one of the following:
Notes:
Organ/space SSIInfection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and infection involves any part of the anatomy (e.g., organs or spaces), other than the incision, which was opened or manipulated during an operation and at least one of the following:
|
Table 2 Operations and Likely Surgical Site Infection (SSI) Pathogens
| Operations | Likely Pathogens |
| Placement of all grafts, prostheses, or implants | Staphylococcus aureus; Coagulase negative Staphylococci (CoNS) |
| Cardiac | Staphylococcus aureus; CoNS |
| Neurosurgery | Staphylococcus aureus; CoNS |
| Breast | Staphylococcus aureus; CoNS |
| Ophthalmic | S. aureus; CoNS; streptococci; gram negative bacilli (GNBs) |
| Orthopedic: Total joint replacement, closed fractures/use of nails, bone plates, other internal fixation devices, functional repair without implant/device, Trauma | Staphylococcus aureus; CoNS; gram-negative bacilli |
| Noncardiac thoracic, Thoracic (lobectomy, pneumonectomy wedge resection, other noncardiac mediastinal procedures), closed tube thoracostomy | Staphylococcus aureus; CoNS; Streptococcus pneumoniae; gram-negative bacilli |
| Vascular | Staphylococcus aureus; CoNS |
| Appendectomy | Gram-negative bacilli; anaerobes |
| Biliary tract | Gram-negative bacilli; anaerobes |
| Colorectal | Gram-negative bacilli; anaerobes |
| Gastroduodenal | GNBs; streptococci; oropharyngeal anaerobes (e.g.,peptostreptococci) |
| Head and neck (major procedures with incision through oropharyngeal mucosa) | Staphylococcus aureus; streptococci; oropharyngeal anaerobes (e.g., peptostreptococci) |
| Obstetric and gynecologic | GNBs; enterococci; group B streptococci; anaerobes |
| Urologic | Gram-negative bacilli |
Investigations
Samples:
- Pus in sterile, wide mouth screw cap containers (if deep seated abscess, collect samples in anaerobic vials also)
- If pus is not available, wound swabs (deep swabbing)
Samples should be taken from the depth of the wound or the advancing edge, avoiding contamination from surrounding skin.
Transport: Immediate transport to the lab
Culture:
- Aerobic: Blood agar, Mac Conkey agar, 37 0 C in air
- Anaerobic: Brain Heart Infusion agar with hemin and vitamin K (anaerobic incubation): 37 0 C, under anaerobic conditions
- Fungal culture (if needed as in obvious contamination with soil etc): SDA with appropriate antibiotics/ BHI-BA with appropriate antibiotics and incubation temperatures (25 and/or 37 degrees)
Resistance pattern of common pathogens (ICMR Data)
Following is the Indian data of AMR from soft tissue infections/SSI
Table 3 Staphylococcus aureus ICMR AMR Data 2014
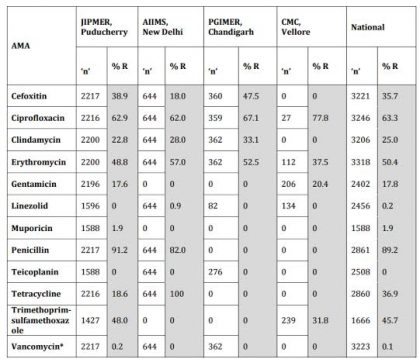
*The 4 numbers listed as Vancomycin Resistant (R) are VISA isolates; No VRSA was isolated during the year 2014 at JIPMER.; Cefoxitin : Surrogate marker for Methicillin.
Table 1Enterobacteriaceae isolates from skin and soft tissue. ICMR data 2014.
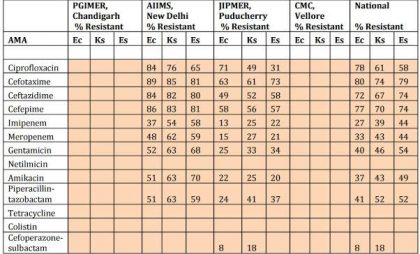
Note: Ec: Escherichia coli; Ks : Klebsiella spp; Es : Enterobacter spp.
Table 2 Pseueudomonas aeruginosa ICMR AMR data 2014
| AMA | PGIMER, Chandigarh 'n' 75 R (%) | AIIMS, New Delhi 'n' 102 R (%) | JIPMER, Puducherry 'n' 113 R (%) | CMC, Vellore 'n' 84 R (%) | National 'n' 374 R % |
| Amikacin | 27 | 49 | 38 | 21 | 35 |
| Aztreonam | 62 | 55 | 30 | 48 | |
| Cefepime | 52 | 57 | 20 | 41 | |
| Cefoparazon e-sulbactam | 39 | 41 | 30 | 38 | |
| Ceftazidime | 64 | 51 | 51 | 23 | 47 |
| Colistin | 34 | 2 | 10 | ||
| Imipenem | 17 | 54 | 48 | 25 | 37 |
| Levofloxacin | 44 | 42 | 23 | 36 | |
| Meropenem | 74 | 41 | 23 | 47 | |
| Netilmicin | 66 | 45 | 22 | 45 | |
| Piperacillin-tazobactam | 44 | 67 | 25 | 46 | |
| Tobramycin | 56 | 43 | 18 | 33 |
Perioperative prophylaxis
Choosing prophylactic antibiotics
- Antibiotics should be chosen on the basis of their effectiveness against the pathogens most likely to be encountered rather than against every possible pathogen. Skin florae (eg, Staphylococcus organisms) are the usual target, so first-generation cephalosporins are recommended (cephalexin, cephalothin) in most studies. Few studies also recommend cefuroxime.
- Patients with a history of anaphylaxis or urticaria after penicillin therapy should not receive prophylaxis with a beta-lactam antibiotic. Vancomycin or clindamycin should be used as alternative.
Timing of prophylactic antibiotics
- Give first dose before incision
- Antibiotics should be administered before an incision is made to ensure that antimicrobial levels in the tissue are adequate and maintained for the duration of the procedure.
- Prophylaxis should be started preoperatively in most circumstances, ideally within 30-60 minutes before incision, except for Vancomy
Route of administration
- Prophylactic antibiotics for surgical procedures should be administered intravenously
Dose selection
- The dose of an antibiotic for prophylaxis is same as for therapy of infection.
Duration
- Continue no longer than 24 hours postoperatively (Except cardiac surgery where data is conflicting)
- Most studies have demonstrated efficacy of postoperative antibiotic prophylaxis for only 12 hours or less. Whenever short and long courses are compared, the shorter course has proven equally effective. A single dose is as effective as multiple doses, and antimicrobial prophylaxis after wound closure is unnecessary.
- Prolonged antibiotic prophylaxis beyond 24 hours is not only ineffective in reducing infections but increases antimicrobial resistance and the risk of colitis due to Clostridium difficile.
Redose for longer surgeries
- Patients undergoing surgery that extends beyond two half-lives of an antibiotic should be re-dosed intraoperatively.
- An additional dose of prophylactic agent is not indicated in adults, unless there is blood loss of up to 1500 ml during surgery or haemodilution of up to 15 ml/kg.
Table 3 Pathogen-specific antimicrobial therapy according to the pathogen isolated
| Surgical Wound Classification | Common Organisms | Antimicrobial prophylaxis |
| Class I/Clean | Gram Positive cocci (S. aureus, CoNS) | None or single perioperative dose of cefuroxime/ cephalexin (Ideally 2 grams) |
| Class II/ Clean-Contaminated | Gram Negative Bacilli Anaerobes S. aureus | 1stLine: Cefazolin or Ampicillinsulbactam or Ceftriaxone (in patients of acute cholecystitis or acute biliary tract infections) Alternative: In case of allergies; if mixture of GP and GN is suspected: Ceftriaxone only if not ESBL clindamycin or vancomycin with cefazolin, aztreonam, gentamicin, or single-dose fluoroquinolone in blactam allergic |
| Class III/Contaminated | Gram Negative Bacilli Anaerobes | 1st line: Cefazolin + Metronidazole 2nd Line: Metronidazole+ Aminoglycoside/ Fluoroquinolone |
| Class IV/Dirty-Infected | Gram Negative Bacilli Anaerobes May be mixed with Gram positive bacteria | 1st Line: Cefazolin + metronidazole, Treatment for infected surgical wounds Ertapenem + Clindamycin + aminoglycoside/aztreonam Or fluoroquinolone+ metronidazole + aminoglycoside/fluoroquinolone |
Table 4 Antibiotics for Treatment of Incisional Surgical Site Infections In culture confirmed cases of SSI/ soft tissue infections, antimicrobials should be based on Lab AST reports
| Surgery | Common organisms | Peri-op antimicrobial prophylaxis |
| Surgery of Intestinal or Genitourinary Tract | Gram Negative Bacilli, anaerobes | 1st Line: Piperacillin-tazobactam 3.375 g every 6 h or 4.5 g every 8 h IV Or Imipenem-cilastatin 500 mg every 6 h IV 2nd Line (as in case of non ESBL organisms) : Ceftriaxone 1 g every 24 h + metronidazole 500 mg every 8 h IV |
| Surgery of trunk or extremity away from axilla or perineum | S. aureus, CoNS | 1st Line: Oxacillin/ nafcillin 2 g every 6 h IV Or Cefazolin 0.5–1 g every 8 h IV 2nd Line: Cefotaxime 500 mg every 6 h IV |
| Surgery of axilla or perineum | S.aureus, GNBs, anaerobes | 1st Line: Metronidazole 500 mg every 8 h IV plus Levofloxacin 750 mg every 24 h 2nd Line: Metronidazole 500 mg every 8 h IV plus Ceftriaxone 1 g every 24 h |
Table 5 Antimicrobial guidelines for treatment of Skin and Soft Tissue Infections
| Clinical Syndrome/ condition | Most likely pathogens | Antibiotic | Comments |
| Impetigo and skin soft-tissue infections | Staphylococci & Streptococci | 1st Line Clindamycin 300-400 mg qid PO Alternative: Amoxicillin-clavulanate 875/125 mg bid po | Local: Mupirocin ointment Apply to lesions bid |
| Erysipelas, Cellulits, Necrotising fasciitis | Streptococci (usually GAS) | Penicillin 2–4 million units X 4–6 h IV or Alternative Clindamycin 600–900 mg X 8 h IV | In penicillin allergic patients: Clindamycin, vancomycin or linezolid, |
| Cutaneous anthrax | Bacillus anthracis | 1st Penicillin G8–12 MU/day IV in divided doses every 4-6 h or Erythromycin 250 mg PO every 6 hours | |
| Necrotizing infections of the skin, fascia, and muscle | Mixed infections | 1st Line Piperacillin-tazobactam + Vancomycin 3.37 g every 6–8 h IV+ 30 mg/kg/d in 2 divided doses Alternative Carbapenems | |
| Water related injuries (water sports etc) | Aeromonas hydrophila Vibrio vulnificus | Doxycycline100 mg every 12 h IV+ ciprofloxacin500 mg every 12 h IV or Ceftriaxone1 to 2 g every 24 h IV | |
| Bubonic plague Diabetic Foot Infections | Yersinia pestis | 1st Line Streptomycin 1 g IM twice per day or Gentamicin 2 mg/kg loading dose, then 1.7 mg/kg/day in 3 divided doses IV Alternative Tetracycline 500 mg po every 6 h | |
| Mild (treated with oral agents) | MSSA; Streptococcus spp | Cloxacillin/ cephalexin | |
| MRSA | Linezolid, Daptomycin, Vancomycin | ||
| Moderate (treated with oral or initial parenteral agent) or severe (treated with parenteral agents) | MSSA; Streptococcus spp; Enterobacteriac eae; obligate anaerobes | Ceftriaxone, Ampicillin/sulbactam, Moxifloxacin, Ertapenem, Tigecycline |
Table 6 Standard Doses of Antimicrobial Agents Active Against Multidrug Resistant Organisms
| Antimicrobial | IV Dose | Comments |
| Vancomycin | 30–60 mg/kg/d in 2–4 divided doses | Target serum trough concentrations of 15–20 μg/mL in severe infections |
| Daptomycin | 4–6 mg/kg/d | Covers VRE, strains nonsusceptible to vancomycin may be cross-resistant to daptomycin |
| Linezolid | 600 mg every 12 h | 100% oral bioavailability; so oral dose same as IV dose. Covers VRE and MRSA |
| Colistin | 5 mg/kg load, then 2.5 mg/kg every 12 h | Nephrotoxic; does not cover gram-positives or anaerobes, Proteus, Serratia, Burkholderia |
Guidelines by Indian Council of Medical Research :
Dr Soumya Swaminathan, Director General, Indian Council of Medical Research Secretary, Department of Health Research

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd