- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Graves disease-induced thrombotic thrombocytopenic purpura: a case report
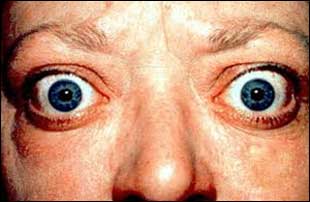
Dr Saira Chaughtai at Internal Medicine Residency Program, Department of Medicine, Hackensack Meridian School of Medicine at Seton Hall University, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, New Jersey, USA and colleagues have reported a rare case of Graves disease-induced thrombotic thrombocytopenic purpura. The case has appeared in the Journal of Medical Case Reports.
Thrombotic thrombocytopenic purpura is an autoimmune disease that carries a high mortality. Very few cases have been reported in the literature that has described a relationship between Graves disease and thrombotic thrombocytopenic purpura. Autoimmune diseases tend to cluster not only in families but also in the same individual.
A 30-year-old African American woman with a history of hypertension, recently diagnosed with hyperthyroidism and receiving methimazole, presented to our hospital with complaints of shortness of breath, dizziness, and weakness that had progressed over 2 days. She had no history of rash, bleeding from any sites of the body, diarrhoea, or vomiting.
Her vital signs showed a blood pressure of 126/96 mmHg, pulse of 109 beats/minute, respirations of 16/minute, a temperature of 98.2 °F, and pulse oxygenation of 100% on room air. On physical examination, she was obese, awake and alert, had marked conjunctival pallor, and had a diffuse and enlarged thyroid gland. She had no petechiae, ecchymosis, bruising, organomegaly, or lymphadenopathy.
Initial laboratory studies showed anemia with hemoglobin of 7.9 g/dl (normal range, 12–16 g/dl), thrombocytopenia with platelets of 4000/μl (140,000-450,000/μl), elevated lactate dehydrogenase of 653 IU/L (91–200 IU/L), haptoglobin < 6 mg/dl (30–225 mg/dl), reticulocyte count 5.31% (0.4–2.5%), high D-dimer of 1725 ng/ml (< 501 ng/ml), high fibrinogen of 633 mg/dl (232–519 mg/dl), and acute renal insufficiency with creatinine 1.20 mg/dl (0.44–1.0 mg/dl) (Table 1). Her peripheral smear showed many schistocytes. The result of her direct Coombs test was negative. Urinalysis showed moderate blood with 8-10 Red blood cells/high power field (0-2 RBCs/hpf) and 100 mg/dl proteins. Her coagulation panel showed prothrombin time and activated partial thromboplastin time of 1.07 and 29 seconds, respectively.
Clinically, the diagnosis of TTP was made, and the patient was transferred to the intensive care unit for close monitoring. She was started on plasmapheresis and intravenous steroids as part of the treatment for TTP. Her ADAMTS13 taken on admission came back a few days later as < 5% (normal, > 61%). Workup for secondary causes of TTP was also done. Her antinuclear antibody was positive at 7.01 (0–0.9), but results of other serological workups, including human immunodeficiency virus, hepatitis profile, anti-double-stranded deoxyribonucleic acid (DNA), cardiolipin antibodies, lupus screen, scleroderma antibodies, and QuantiFERON test (Qiagen, Germantown, MD, USA), were negative. She had positive centromere antibodies, but clinically she had no signs of CREST syndrome (calcinosis cutis, Raynaud phenomenon, oesophageal dysfunction, sclerodactyly, and telangiectasia) or scleroderma. Her complement levels of C3 and C4 were found to be normal.
On further query, the patient reported that her mother had had Graves disease and her twin sister also had had Graves disease and had died of cardiac arrest at the age of 24 in the setting of thyrotoxicosis. The patient’s outpatient records 4 months prior to admission showed a free T4 of 7.7 ng/dl (0.5–1.26 ng/dl) and a thyroid-stimulating hormone (TSH) < 0.005 mIU/ml (0.3–4.5 mIU/ml). Outpatient ultrasound showed an enlarged thyroid with increased background vascularity. The patient’s platelet count was 248,000/μl (140,000–450,000/μl) at that time. She was started on methimazole and was advised to follow up with an endocrinologist, but she did not comply. No autoantibody levels were measured at that time, and she did not have an uptake scan.
On admission, her TSH was found to be 0.528 IU/ml (0.3–4.5 mIU/ml), free T4 was 3.93 ng/dl (0.5–1.26 ng/dl), total T4 was 21.23 μg/dl (5.28–9.27 μg/dl), and free T3 was 7.0 pg/ml (2.28–3.96 pg/ml). Her TSH receptor antibody and thyroid-stimulating immunoglobulin were found to be normal with a positive anti-thyroid peroxidase antibody. Unfortunately, these were drawn following several sessions of plasmapheresis; therefore, the absence or presence of thyroid autoimmune antibodies was not thought to be reliable for the diagnosis of autoimmune thyroid disease. The patient’s Graves disease diagnosis was based on thyromegaly, glandular hypervascularity, and lack of biochemical improvement despite over 4 months of thionamide therapy. In preparation for thyroidectomy, she was treated with propylthiouracil, potassium iodide oral solution, cholestyramine, prednisone, and propranolol. Her thyroid pathology revealed a diffuse thyrotoxic goitre with patchy chronic inflammation.
She was given multiple sessions of plasmapheresis, along with a high dose of steroids, cyclophosphamide, and rituximab. She initially showed marked improvement in her platelet count; however, her hospital course was complicated by hospital-acquired pneumonia possibly provoked by severe immunodeficiency, sepsis, and acute respiratory distress syndrome (ARDS) with ventilator-dependent respiratory failure requiring rotoproning, and her course was further complicated by hemothorax requiring chest tube placement. All these complications led to a subsequent decline in her platelet count. A new medication recently approved for acquired TTP called caplacizumab, a monoclonal antibody to vWF, was considered in her management. Her platelets stabilized at approximately 50,000/μl (140,000–450,000/μl), and her ADAMTS13 activity normalized, so the caplacizumab was not administered. However, due to overwhelming ARDS, her hypoxemia drove her heart into pulseless electrical activity. Despite multiple rounds of cardiopulmonary resuscitation, she died.
Journal of Medical Case Reports
For more details click on the link: https://doi.org/10.1186/s13256-019-2307-1

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd