- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Cluster headache : New Treatment
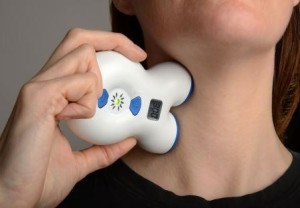
Electrocore, a neuroscience and technology company has come out with a Hand-held, non-invasive, easy-to-use device provides new option for the patients suffering from extremely debilitating headache disorders including Migraine and Custer Headache.
The device called gammaCore transmits a mild electrical stimulation to the vagus nerve through the skin, resulting in a reduction of pain. Designed as a portable, easy-to-use technology, gammaCore can be self-administered by patients as needed to provide relief for the treatment of pain associated with episodic cluster headache without the potential side effects associated with standard of care. When placed on a patient's neck over the vagus nerve, gammaCore stimulates the nerve's afferent fibers resulting in the modification of pain signals.
"Cluster headache is a rare, debilitating and difficult to treat disorder with few effective acute therapies," said Stephen Silberstein, MD, Director, Headache Center, Jefferson University, Philadelphia, PA. "The FDA release of gammaCore is an important advance in the treatment of the pain associated with cluster headache. It is a way for patients to treat their symptoms as often as they need to use the device. It does not have the side effects or dose limitations of commonly prescribed treatments or the need for invasive implantation procedures, which can be inconvenient, costly and high-risk."
The FDA release of gammaCore is based on subgroup analyses from two trials in the ACT (Non–Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache) clinical trial program evaluating the safety and efficacy of gammaCore for the acute treatment of episodic cluster headache. Both trials (ACT1 and ACT2) were prospective, double-blind, placebo-controlled, randomized studies evaluating the use of gammaCore versus placebo. Results from ACT1, evaluating 85 patients with episodic cluster headache, found that 34.2% of patients experienced a reduction in pain from episodic cluster headache (defined as the percentage of patients who reported mild or no pain 15 minutes after treatment initiation with gammaCore for the first treated cluster headache attack in the study; use of rescue medication within 60 minutes was considered a treatment failure) compared to 10.6% in patients treated with placebo (p=0.008).
Results from ACT2, evaluating 182 attacks in 27 patients with episodic cluster headache, also found that a significantly higher percentage of attacks were pain-free (defined as pain-free at 15 minutes after the onset of pain from cluster headache with no use of rescue medication through the 30-minute treatment period) in patients treated with gammaCore (47.5%) versus placebo (6.2%; p=0.003).i
In both trials, gammaCore was found to be safe and well-tolerated, with the majority of adverse events (AEs) being mild and transient and occurring during the time of active treatment. It is hoped that gammaCore shall come very handy in acute and/or prophylactic treatment of primary headache (Migraine, Cluster Headache, and Hemicrania Continua).
for further reading log on to
Silberstein, S. D., Mechtler, L. L., Kudrow, D. B., Calhoun, A. H., McClure, C., Saper, J. R., Liebler, E. J., Rubenstein Engel, E., Tepper, S. J. and on behalf of the ACT1 Study Group (2016), Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache: The Journal of Head and Face Pain, 56: 1317–1332. doi:10.1111/head.12896
The device called gammaCore transmits a mild electrical stimulation to the vagus nerve through the skin, resulting in a reduction of pain. Designed as a portable, easy-to-use technology, gammaCore can be self-administered by patients as needed to provide relief for the treatment of pain associated with episodic cluster headache without the potential side effects associated with standard of care. When placed on a patient's neck over the vagus nerve, gammaCore stimulates the nerve's afferent fibers resulting in the modification of pain signals.
"Cluster headache is a rare, debilitating and difficult to treat disorder with few effective acute therapies," said Stephen Silberstein, MD, Director, Headache Center, Jefferson University, Philadelphia, PA. "The FDA release of gammaCore is an important advance in the treatment of the pain associated with cluster headache. It is a way for patients to treat their symptoms as often as they need to use the device. It does not have the side effects or dose limitations of commonly prescribed treatments or the need for invasive implantation procedures, which can be inconvenient, costly and high-risk."
The FDA release of gammaCore is based on subgroup analyses from two trials in the ACT (Non–Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache) clinical trial program evaluating the safety and efficacy of gammaCore for the acute treatment of episodic cluster headache. Both trials (ACT1 and ACT2) were prospective, double-blind, placebo-controlled, randomized studies evaluating the use of gammaCore versus placebo. Results from ACT1, evaluating 85 patients with episodic cluster headache, found that 34.2% of patients experienced a reduction in pain from episodic cluster headache (defined as the percentage of patients who reported mild or no pain 15 minutes after treatment initiation with gammaCore for the first treated cluster headache attack in the study; use of rescue medication within 60 minutes was considered a treatment failure) compared to 10.6% in patients treated with placebo (p=0.008).
Results from ACT2, evaluating 182 attacks in 27 patients with episodic cluster headache, also found that a significantly higher percentage of attacks were pain-free (defined as pain-free at 15 minutes after the onset of pain from cluster headache with no use of rescue medication through the 30-minute treatment period) in patients treated with gammaCore (47.5%) versus placebo (6.2%; p=0.003).i
In both trials, gammaCore was found to be safe and well-tolerated, with the majority of adverse events (AEs) being mild and transient and occurring during the time of active treatment. It is hoped that gammaCore shall come very handy in acute and/or prophylactic treatment of primary headache (Migraine, Cluster Headache, and Hemicrania Continua).
for further reading log on to
Silberstein, S. D., Mechtler, L. L., Kudrow, D. B., Calhoun, A. H., McClure, C., Saper, J. R., Liebler, E. J., Rubenstein Engel, E., Tepper, S. J. and on behalf of the ACT1 Study Group (2016), Non-Invasive Vagus Nerve Stimulation for the ACute Treatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache: The Journal of Head and Face Pain, 56: 1317–1332. doi:10.1111/head.12896
Cluster HeadachegammaCoreJournal of Head and Face PainNerve Stimulation TherapyNon-Invasive Nerve Stimulation TherapySuicide headacheVagus Nerve Stimulation
Next Story
NO DATA FOUND

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd