- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
First ever smartphone controlled device gets FDA approval for migraine management
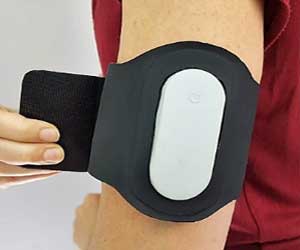
The US Food and Drug Administration has now approved worlds first smartphone-controlled electroceutical Nerivio Migra® with Remote Electrical Neuromodulation devised for the treatment of acute migraine.
Nerivio Migra® is the first-in-category product which uses smartphone-controlled electrical pulses to create a Conditioned Pain Modulation (CPM) response when placed on the upper arm
"The clinical data of this innovative therapeutic device is of very high quality," commented Professor Messoud Ashina, Danish Headache Center, president-elect of the International Headache Society. "It indicates that the device can provide patients with significant relief of pain and other migraine symptoms without the side effects presented by drugs."
The FDA approval is based on the results of a prospective, randomized, double-blind, placebo-controlled, multi-center pivotal study, where 252 patients from 12 clinics used the non-invasive wearable to treat their migraine attacks.
"This study followed the latest edition of the guidelines from the International Headache Society for controlled trials of acute treatment of migraine attacks in adults," said Dr. Brian Grosberg, MD, director of the Hartford Healthcare Headache Center in Connecticut, who served as the lead Principal Investigator (PI) of the study. "The results of the study demonstrate a high efficacy ratio for single as well as multiple attacks, both at two and 48 hours after treatment," explained Dr. Grosberg, who was a co-author of an article describing the study, released earlier this month in Headache: The Journal of Head and Face Pain published by Wiley Periodicals, Inc. on behalf of the American Headache Society.
Nerivio Migra®, a first-in-category product, is placed on the upper arm (not the head or neck) and uses smartphone-controlled electronic pulses to create a Conditioned Pain Modulation (CPM) response. Nerivio Migra® is indicated for acute treatment of migraine with or without aura in adult patients who do not have chronic migraine.
"While the company is preparing to launch the Nerivio Migra® in the United States market later this year at an affordable price, we remain committed to continuing our clinical development, expanding the use of remote electrical neuromodulation therapy for additional indications," said Alon Ironi, CEO and co-founder of Theranica. "We have identified at least 7 different painful conditions that may be relieved by this non-invasive, drug-free technology after appropriate clinical development."
Migraine is the third most common disease in the world, with an estimated global prevalence of 14.7% of the world's population. Migraine results in $17 billion of annual health costs in the U.S. alone. In a study conducted with 2,444 people suffering from migraine, two-thirds delayed or avoided taking their prescription medications due to concerns about adverse side effects, and 79% sought products with similar efficacy but fewer side effects.
"Physicians who treat people with migraine are both patient-centered and science-driven," said Prof. Stephen Silberstein, director of the Headache Center at the Jefferson University Hospital in Philadelphia, a member of the medical advisory board of Theranica. "Over the last 20 years my colleagues and I have used triptans and ergots for acute migraine treatment. There is a large unmet need for new treatments in this population when these medications are not effective, are contra-indicated, or have non-tolerable side effects. In addition, triptans and most current acute migraine medications, including over-the-counter drugs indicated for migraine, are associated with medication-overuse headache (MOH), which is associated with increased frequency of migraine attacks, and often results in chronic migraine. This new innovative FDA-authorized treatment is an important alternative to help our patients control this debilitating condition."
Theranica Bioelectronics, founded in 2016, is dedicated to combining advanced neuromodulation therapy with modern wireless technology to develop proprietary electroceuticals that address prevalent medical conditions and diseases. Nerivio Migra®, Theranica's first FDA authorized to the market device is a low-cost, low side effect wearable for the acute treatment of migraine. Theranica will continue to use its proprietary technology to develop additional solutions to other painful disorders.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd