- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
First Drug approved for unresectable rare adrenal tumors
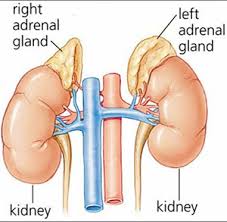
The U.S. Food and Drug Administration has approved Azedra (iobenguane I 131) injection for intravenous use. Azedra is indicated for the treatment of adults and adolescents aged 12 and older with rare tumors of the adrenal gland (pheochromocytoma or paraganglioma) that cannot be surgically removed (unresectable), have spread beyond the original tumor site and require systemic anticancer therapy.
This is the first FDA-approved drug for the treatment of rare adrenal tumors. The approval for Azedra was granted to Progenics Pharmaceuticals.
Pheochromocytomas are rare tumors of the adrenal glands. These glands are located right above the kidneys and make hormones including stress hormones (epinephrine and norepinephrine). Pheochromocytomas increase the production of these hormones, leading to hypertension (high blood pressure) and symptoms such as headaches, irritability, sweating, rapid heart rate, nausea, vomiting, weight loss, weakness, chest pain or anxiety. When this type of tumor occurs outside the adrenal gland, it is called a paraganglioma.
The FDA granted this application Fast Track, Breakthrough Therapy and Priority Review designations. Azedra also received Orphan Drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
“Many patients with these ultra-rare cancers can be treated with surgery or local therapies, but there are no effective systemic treatments for patients who experience tumor-related symptoms such as high blood pressure,” Richard Pazdur, director of the FDA’s Oncology Center of Excellence, said in a press release. “Patients will now have an approved therapy that has been shown to decrease the need for blood pressure medication and reduce tumor size in some patients.”
Also Read:Fortis doctors remove whooping 11.5kg Adrenal Tumor from patient with BMI- 48
The approval was based on a single-arm, open-label, clinical trial in 68 patients that measured the number of patients who experienced a 50 percent or greater reduction of all antihypertensive medications lasting for at least six months. This endpoint was supported by the secondary endpoint, overall tumor response measured by traditional imaging criteria.
Key Findings of the Trial:
- The study met the primary endpoint, with 17 (25 percent) of the 68 evaluable patients experiencing a 50 percent or greater reduction of all antihypertensive medication for at least six months.
- Overall tumor response was achieved in 15 (22 percent) of the patients studied.
- The most common severe side effects reported by patients receiving Azedra in clinical trials included low levels of white blood cells (lymphopenia), abnormally low count of a type of white blood cells (neutropenia), low blood platelet count (thrombocytopenia), fatigue, anemia, increased international normalized ratio (a laboratory test which measures blood clotting), nausea, dizziness, hypertension and vomiting.
Important Safety Information
As it is a radioactive therapeutic agent, Azedra includes a warning about radiation exposure to patients and family members, which should be minimized while the patient is receiving Azedra. The risk of radiation exposure is greater in pediatric patients.
Other warnings and precautions include a risk of lower levels of blood cells (myelosuppression), underactive thyroid, elevations in blood pressure, renal failure or kidney injury and inflammation of lung tissue (pneumonitis). Myelodysplastic syndrome and acute leukemias, which are cancers of the blood and bone marrow, were observed in patients who received Azedra, and the magnitude of this risk will continue to be studied. Azedra can cause harm to a developing fetus; women should be advised of the potential risk to the fetus and to use effective contraception after receiving Azedra. Radiation exposure associated with Azedra may cause infertility in males and females.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd