- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
FDA clears world's first total-body imaging 3D scanner
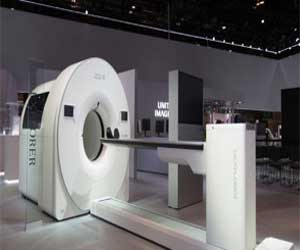
The US Food & Drug Administration (FDA) has cleared uEXPLORER total-body scanner, making it commercially available, announced medical equipment company United Imaging. The scanner is the world's first medical imaging 3D scanner that can capture the whole human body at 1-bed position.
The technology combines X-ray Computed Tomography (CT) with Positron Emission Tomography (PET) allowing for imaging of the entire body at the same time. It can be used in different ways such as tracking disease progression, researching new treatments, and improving diagnostics. For example, uEXPLORER will be used to better visualize cancer that has spread beyond a single tumor site. Researchers also envision using the scanner to measure blood flow across the entire body and study inflammation, infections and immunological/metabolic disorder.
“This is truly a game-changing, revolutionary day for the advancement of medical technology,” Jeffrey M. Bundy, Chief Executive Officer of UIH Solutions, said in a press release. “The human images the uEXPLORER produces have been nothing short of astonishing, especially when one considers the very short acquisition times. This device will bring us into completely new areas of discussion with our customers, as we jointly re-think the role of PET in the North American healthcare system. We are thrilled to be able to start sharing it widely in the US.”
“As we had originally envisioned the uEXPLORER will have a profound impact on clinical research and patient care,” said Dr. Cherry, professor in the UC Davis Department of Biomedical Engineering and co-developer of uEXPLORER. Dr. Badawi, Chief of Nuclear Medicine at UC Davis added: “With this device, we will be able to ask and answer an array of new biological questions – that’s truly incredible for the future of healthcare for all of us.”
United Imaging developed uEXPLORER based on its latest hardware and software platform, focusing on not only delivering the target performances but also making such a large system more robust and user-friendly, resulting in technology that produces higher-quality diagnostic PET scans than have ever been possible. The first images from scans of humans using the new device were unveiled at the 104th RSNA Scientific Assembly and Annual Meeting, the official meeting of the Radiological Society of North America (RSNA), in Chicago.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd