- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
FDA clears KardioMobile, a personal ECG device to detect rhythm disorders
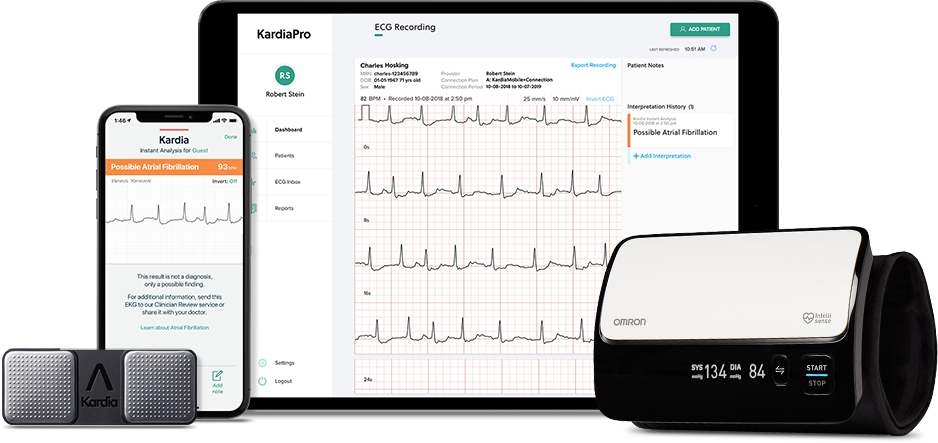
The Food and Drug Administration (FDA) has cleared KardiaMobile which is a personal ECG device that can be used for detecting rhythm disorders like bradycardia and tachycardia.
KardiaMobile detects Atrial Fibrillation, Bradycardia, Tachycardia, as well as Normal Sinus Rhythm. And KardiaMobile powers KardiaPro, the free Remote Patient Monitoring platform that allows your practice to harness your patients’ reports simply, without data overload.
While first generation monitoring systems are challenging to navigate and difficult to monetize, KardiaPro presents data in a simple, intuitive format designed to conform with CPT reimbursement code requirements.
KardiaPro is a web‑based portal that presents medical‑grade patient data from the most clinically validated and widely used ECG and blood pressure monitoring devices in the world. KardiaPro is used by providers to support clinical decision making. Actionable data is organized and triaged based on customized practice parameters for efficiency. KardiaPro is completely free to your practice with no hardware to buy or sell.
- KardiaPro allows physicians to deliver a higher quality of care to more patients.
- Physicians are able to avoid unneeded in-person visits.
- Physicians are able to spend more time with patients who truly need their expertise.
This portable EKG reader, which works with smartphones and tablets, was first cleared by the FDA in 2017 for detection of normal sinus rhythm and atrial fibrillation. When paired with the Kardia app, the device now provides instant analysis for 3 of the most common cardiac arrhythmias. Through the KardioPro platform, clinicians are able to remotely monitor patients, which the Company believes helps support clinical decision making and may help reduce unneeded in-person visits.
“Until today, patients have been frustrated when devices label their ECG reading as ‘unclassified’ or ‘inconclusive.’ Starting today, KardiaMobile is the first personal ECG device that can begin to materially reduce the number of those determinations,” said AliveCor Chief Executive Officer, Ira Bahr. “Critically, KardiaMobile is also the only personal ECG that can detect atrial fibrillation at heart rates above 120, and heart rates below 40.”

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd