- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
FDA clears 14-Day version of ambulatory cardiac patch monitoring

Food and Drug Administration has granted clearance to the 14-Day version of the Carnation Ambulatory Monitor ("CAM™"), the industry's only P-wave centric™ambulatory cardiac patch monitor and arrhythmia detection device. The clearance was received by Bardy Diagnostics, Inc., ("BardyDx"), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions.
The U.S. Food and Drug Administration ("FDA") has granted 510(k) clearance for the 14-Day version of the Carnation Ambulatory Monitor ("CAM™"). Developed to provide clinicians greater flexibility in cardiac monitoring over a longer period, the award-winning CAM patch will now be offered in a 14-day extended wear version that utilizes the same innovative P-wave focused technology that powers the existing 2-Day and 7-Day CAM product lines.
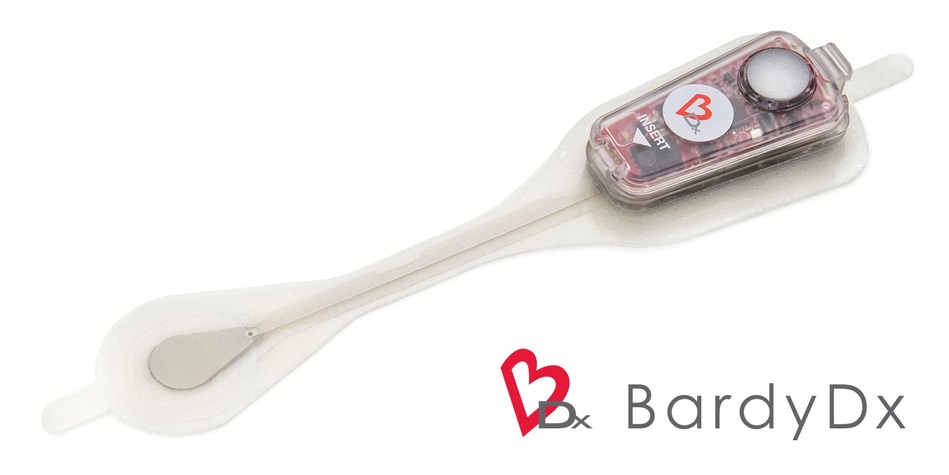
BardyDx Carnation Ambulatory Monitor (CAM™) - P-wave centric™ ambulatory cardiac patch monitoring and arrhythmia detection (PRNewsfoto/Bardy Diagnostics, Inc.)
"Comfortable dermal ECG recordings that focus on the P-wave for up to 14 days carry the potential to minimize use of costly additional rhythm diagnostic tools," said Gust H. Bardy, MD, Founder and Chief Executive Officer of BardyDx. "We are excited to receive clearance from the FDA to enable clinicians the option to monitor longer and anticipate incremental detection of accurate, medically actionable data to improve patient management."
The CAM patch's breakthrough technology reliably detects and records the P-wave, a small amplitude signal of the ECG originating in the atrium that is essential to accurate arrhythmia diagnosis and patient management. Unlike other commercially available patches that include licensed, acquired, or off-the-shelf
The new 14-Day CAM patch enables up to double the duration of P-wave optimized detection and monitoring over the current 7-Day CAM patch, potentially discovering additional, less-frequent arrhythmias. The clinical value of P-wave focused detection was highlighted in the American Heart Journal that published the results of a head-to-head comparison with the iRhythm Zio® XT patch. The study, "Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity," (Am Heart J 2018; 203:109-117) concluded that the BardyDx CAM patch identified 40% more arrhythmias and resulted in better, more informed clinical decision-making in 41% of patients compared to the iRhythm Zio XT patch.
In addition, a preceding study comparing the BardyDx CAM patch and a traditional Holter monitor, entitled "Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours," (Am Heart J 2017; 185:65-73) showed a four times increase in arrhythmia detection using the CAM patch, including arrhythmias missed or incorrectly identified using the Holter monitor. The study concluded that the CAM patch offered significantly improved rhythm diagnosis and avoided inaccurate diagnoses made using a Holter monitor.
"Combining the excellence of our proven P-wave technology with the capability of longer duration monitoring produces an unmatched cardiac monitoring solution," said Ken Nelson, BardyDx Chief Commercial Officer. "We are excited to offer a 14-Day CAM patch that truly provides value for clinicians and patients alike."
The innovative P-wave centric CAM patch continues to distinguish itself in an increasingly crowded market of cardiac monitoring patch entrants. Recently, BardyDx was named the winner of the 2019 MedTech Breakthrough Award for Best New Diagnostic Technology and the winner of the 2019 Frost & Sullivan Award for Technology Innovation in Remote Cardiac Monitoring. Also, BardyDx has been recently named the winner of the Impact Pediatric Health Competition hosted by the nation's leading pediatric healthcare institutions at SXSW 2019 for the CAM's unique pediatric-friendly design and potential to address significant unmet needs in pediatric healthcare. In addition, BardyDx was also selected as the winner of the 2018 Fierce Innovation Life Sciences Award for Medical Device Innovation from the leading industry publisher of FierceBiotech & FiercePharm
For more information, please visit www.bardydx.com.
14-Day versionambulatory cardiac patchBardyDxCAMCAM patchcardiac monitoring technologiesCarnation Ambulatory MonitorclearanceCustomdata solutionsFood and Drug AdministrationIncP-wave centric
Next Story
NO DATA FOUND

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd