- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
FDA approves Control-IQ artificial pancreas system
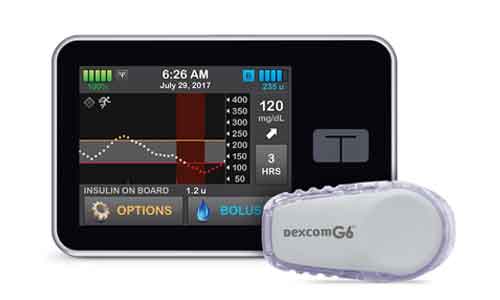
The U.S. Food and Drug Administration has approved an artificial pancreas system that automatically monitors and regulates blood sugar levels. The system is based on technology from the University of Virginia Center for Diabetes Technology.
The artificial pancreas system called Control-IQ is being manufactured by Tandem Diabetes Care. It tracks blood sugar levels with a continuous glucose monitor (Dexcom G6 CGM) and automatically delivers the hormone insulin as and when needed. The system frees people from testing their blood sugar levels multiple times a day by fingerstick, and from delivery of insulin by multiple daily injections.
FDA approval follows results from a multicenter clinical trial published in the New England Journal of Medicine that found the new artificial pancreas system was more effective than existing treatments at controlling blood sugar levels in people with type 1 diabetes. The study showed that the system improved blood sugar control throughout the day and overnight.
“We are excited that our decade-long research, which recently culminated in a large-scale clinical trial published in the New England Journal of Medicine, has been successfully translated to the clinical practice,” said Boris Kovatchev, PhD, director of the UVA centre. “This is a new-generation interoperable automated glucose control system, which allows seamless integration of a continuous glucose sensor, insulin pump, and a smart control algorithm”.
Better Managing Type 1 Diabetes
The pump is programmed with an algorithm developed at UVA that uses glucose monitoring information to adjust the recipient’s insulin dose automatically.
During the multicenter clinical trial , 168 participants age 14 or older with type 1 diabetes were randomly assigned to use either the artificial pancreas system or sensor-augmented pump (SAP) therapy with a continuous glucose monitor and insulin pump that did not automatically adjust insulin throughout the day.
The researchers found that users of the artificial pancreas system significantly increased the amount of time with their blood glucose levels in the target range, while the time in range in the SAP group remained unchanged over six months. Artificial pancreas users also showed improvements in several diabetes control measures, including time with high and low blood glucose, compared with the SAP group. During the study, no severe hypoglycemia events occurred in either group. Diabetic ketoacidosis, in which the blood becomes too acidic, occurred in one participant in the artificial pancreas group due to a problem with equipment that delivers insulin from the pump.
Development of the Artificial Pancreas
Kovatchev thanked the numerous people and groups that have supported the development of the artificial pancreas system, including Paul and Diane Manning of Charlottesville; the Frederick Banting Foundation of Richmond; the JDRF’s Artificial Pancreas Project; the UVA Strategic Investment Fund Project No. 88; and the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases.“We are thankful to everyone that supported the development of the artificial pancreas,” said UVA endocrinologist Sue Brown, MD, the lead investigator of the clinical trial that tested Control-IQ. “Artificial pancreas systems can help people with type 1 diabetes improve blood sugar control while also making it easier for them to manage their blood sugar levels.”
Journal information: New England Journal of Medicine, 2019; DOI: 10.1056/NEJMoa1907863

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd