- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
ESC guidelines for diagnosis and management of peripartum cardiomyopathy
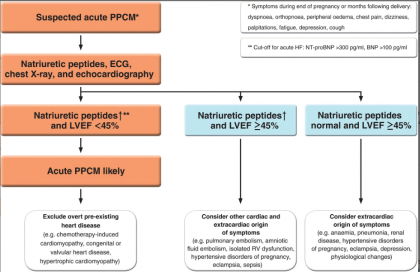
The heart failure association of the European Society of Cardiology has released new guidelines for pathophysiology, diagnosis, and management of peripartum cardiomyopathy. The guideline was published in the European Journal of Heart Failure
Cardiomyopathies are not very common diseases but may cause severe complications, making a substantial contribution to maternal morbidity and mortality during pregnancy, in the immediate peripartum period, and up to months later. Heart failure due to PPCM provides a challenge for treating physicians as PPCM presentation may vary from subtle signs and symptoms to severe acute heart failure, pulmonary oedema and/or cardiogenic shock.
In this position statement, the scientists of heart failure association of the ESC have summarized the current knowledge about pathophysiology and clinical best practice in the management of PPCM patients.
The guideline defines peripartum cardiomyopathy as
- Heart failure secondary to left ventricular systolic dysfuntion with a LVEF < 45%
- Occurrence towards the end of pregnancy or in the months following delivery (mostly in the month following delivery)
- No other identifiable cause of heart failure
Pathophysiology
The aetiology of PPCM is dubious. Possible factors leading to PPCM include genetic predisposition, low selenium levels, viral infections, stress‐activated cytokines, inflammation, autoimmune reaction, a pathological response to hemodynamic stress, unbalanced oxidative stress, and induction of antiangiogenic factors. Particularly, the oxidative stress‐mediated cleavage of the hormone prolactin into a smaller antiangiogenic subfragment, 16‐kDa prolactin, may drive PPCM by inducing endothelial damage, the guideline stated.
Genetic aspects
- PPCM is often difficult to differentiate from genetically transmitted dilated cardiomyopathy (DCM) which may manifest during early adulthood. recent observations support the notion that around 15–20% of patients with peripartum heart failure carry mutations known to induce cardiomyopathies, i.e. in genes like titin, beta‐myosin heavy chain, myosin‐binding protein C (MYBPC3), lamin A/C or sodium voltage‐gated channel alpha subunit 5 (SCN5A). Further investigation is needed regarding mutations or polymorphisms in genes regulating metabolism, oxidative stress response, angiogenesis and the immune system as well as the higher frequency of PPCM in women of African ancestry.
- Genetic testing may be considered in PPCM, in particular in those patients with a positive familial history.
Differential diagnoses of peripartum cardiomyopathy
| History | Onset | Biomarkers | Echocardiography/cardiac MRI | Differentiation from PPCM | |
|---|---|---|---|---|---|
| PPCM | No known cardiac disease, no HF signs and/or symptoms prior pregnancy | Towards the end of pregnancy and the months following delivery | Elevated natriuretic peptides | Reduced systolic LV function, LVEF < 45% | |
| Myocarditis | Prior viral infection (e.g. respiratory) | Acute or subacute onset after viral infection | Elevated troponin, elevated CRP | Normal or reduced systolic LV function, typical myocardial late gadolinium enhancement pattern, pericardial effusion | Cardiac MRI (LE pattern), myocardial biopsy |
| Pre‐existing idiopathic/ familial dilated or acquired cardiomyopathy | HF signs and/or symptoms and/or known heart disease prior pregnancy | During second trimester of pregnancy | Elevated natriuretic peptides | Reduced systolic LV function, RV dysfunction possible, typical myocardial LE pattern (DCM) | History, echocardiography, cardiac MRI (LE pattern) |
| Takotsubo syndrome | Chest pain, very stressful delivery or emergency due to foetal complications | Acute onset, during delivery or immediately after delivery | Elevated natriuretic peptides | Regional wall motion abnormalities with typical anatomical patterns | History, echocardiography |
| Pregnancy‐associated myocardial infarction | Chest pain, epigastric pain | Acute onset, during pregnancy or immediately after delivery | Elevated troponin | Regional wall motion abnormalities, ischaemic myocardial scar | History, ECG, coronary angiography, cardiac MRI (LE pattern) |
| Pulmonary embolism | Chest pain, unilateral leg swelling, acute dyspnoea | Acute onset during pregnancy or after delivery | Elevated natriuretic peptides and/or troponin, elevated D‐dimer | RV dysfunction, RV dilatation, LV function usually normal | Computed tomography, VQ scan |
| Amniotic fluid embolism | Chest pain during/immediately after delivery, acute dyspnoea | Acute onset during delivery or immediately after delivery | Elevated natriuretic peptides possible | Reduced RV systolic function, RV dilatation | History, echocardiography |
| Hypertensive heart disease/severe pre‐eclampsia | Pre‐existing or new‐onset hypertension, proteinuria | During second trimester of pregnancy | Elevated natriuretic peptides | LV hypertrophy, diastolic dysfunction, transient LV dysfunction | History, echocardiography |
| Hypertrophic cardiomyopathy | Familial predisposition | During second trimester of pregnancy | Elevated natriuretic peptides | LV hypertrophy, typical myocardial late enhancement pattern, LVOTO (HOCM) | History, echocardiography, cardiac MRI (LE pattern) |
| HIV/AIDS cardiomyopathy | HIV infection, AIDS | During second trimester of pregnancy | Elevated natriuretic peptides | Reduced systolic LV function, LV/RV often not dilated | HIV serology/test |
| Pre‐existing (unknown) congenital heart disease | HF signs and/or symptoms prior pregnancy, known heart disease, prior cardiac surgery | During second trimester of pregnancy | Elevated natriuretic peptides | (Corrected) congenital heart defects, cardiac shunts | History, echocardiography |
| Pre‐existing valvular heart disease | HF signs and/or symptoms prior pregnancy, known heart disease | During second trimester of pregnancy | Elevated natriuretic peptides | Valvular stenosis or regurgitation, prosthetic heart valves | History, echocardiography |
PPCM should be suspected in all women with a delayed return to the pre‐pregnancy state. Table 2 summarizes the diagnostic tests that are recommended for the diagnosis of PPCM at initial diagnosis and at follow‐up visits.
| Clinical examination | ECG | Natriuretic peptides | Echocardiography | Chest X‐ray | Cardiac MRI | CT scan | Coronary angiography | |
|---|---|---|---|---|---|---|---|---|
| Diagnosis of PPCM | X | X | X | X | X | (X)b | (X)b | (X)b |
| 4‐6 weeks after diagnosis | X | X | X | X | (X)b | (X)b | (X)b | |
| 3 months after diagnosis | X | X | Xa | X | ||||
| 6 months after diagnosis | X | X | Xa | X | (X)b | |||
| 12 months after diagnosis | X | X | Xa | X | ||||
| 18 months after diagnosis | X | X | Xa | X | ||||
| Annually for at least 5 years after | X | X | Xa | X | ||||
| diagnosis (especially if not fully recovered) |
- Generally, an individual approach is recommended depending on the severity of the disease and/or potential differential diagnoses.
- CT, computed tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging; PPCM, peripartum cardiomyopathy.
- a May be considered depending on costs and local availability.
- b May be considered depending on the clinical presentation and/or differential diagnoses.
Electrocardiogram
- An ECG should be performed in all patients with suspected PPCM because it is safe, inexpensive and may help distinguish PPCM from other causes of symptoms. Although there is no specific ECG pattern for PPCM, at initial evaluation, the ECG is rarely normal and repolarization abnormalities are common.
- Left bundle branch block may be an indirect sign for cardiomyopathy and structural heart disease should be ruled out in these women.
- A recent study identified a long QTc interval at baseline which was found in almost 50% of the patients, and tachycardia as predictors of poor outcome in PPCM.
Biomarkers
- Concerning diagnostic properties of natriuretic peptides, one should keep in mind that B‐type natriuretic peptide (BNP)/N‐terminal proBNP (NT‐proBNP) levels are not or only slightly elevated in normal pregnancy.
Cardiac imaging
- Echocardiography is indicated as soon as possible in all cases of suspected PPCM to confirm the diagnosis, assess concomitant or pre‐existing cardiac disease, exclude complications of PPCM (e.g. LV thrombus) and obtain prognostic information (for example LVEF and pulmonary hypertension).
- After stabilization, magnetic resonance imaging may provide a more accurate evaluation of cardiac structure and function, and can sometimes be helpful if there is high suspicion for another diagnosis such as arrhythmogenic right ventricular cardiomyopathy and myocarditis.
- The incremental value of cardiac magnetic resonance imaging in addition to echocardiography is uncertain.
- Administration of gadolinium to assess late enhancement should be avoided until after delivery due to the increased risk of stillbirth, neonatal death, and rheumatological, inflammatory, or infiltrative skin conditions.
Endomyocardial biopsy
- Endomyocardial biopsy adds limited diagnostic or prognostic information in PPCM.
- It may be used to exclude acute myocarditis after delivery, reveal significant viral presence, and exclude rare autoimmune myocarditis, storage or metabolic disease.
Management
- For rapid diagnosis and decision making in all pregnant women with acute heart failure, a pre‐specified management algorithm and the establishment of a multidisciplinary team is crucial.
- Multidisciplinary care includes cardiologists, intensivists, obstetricians, neonatologists, anaesthetists and cardiac surgeons.

- If a patient is in cardiogenic shock/dependent on inotropes, she should be transferred immediately to an advanced heart failure centre where mechanical circulatory support (MCS), ventricular assist devices (VAD), and transplant consult teams are available.
- Experimental data and a study in PPCM patients indicated that patients with PPCM may be especially sensitive to toxic effects of beta‐adrenergic receptor stimulation which should be avoided whenever possible.
- Norepinephrine is indicated to restore blood pressure, and levosimendan may be considered, however the only (small) randomized clinical trial in PPCM patients did not show a beneficial effect on outcome.
- The teratogenic effects of inotropic support and vasopressors in humans are unknown but their use may be necessary.
- In PPCM patients with severely reduced LV function and/or cardiogenic shock, VAD implantation as bridge to recovery or transplantation can be necessary (2–7% of PPCM patients).
- It is important to note, however, that a significant proportion of PPCM patients improve or normalize their LV function over the first 6 months after diagnosis, which must be considered when decisions are made.
- Short‐term assist devices, e.g. microaxial pump, Centrimag or venoarterial extracorporeal membrane oxygenation (ECMO), may be required.
- Long‐term assist devices with left VAD or biventricular VAD can be implanted and some have been explanted after recovery.
- Due to the toxic effects of beta‐adrenergic agonists specifically in PPCM, MCS may be considered with a lower threshold than in other patients with inotrope‐dependent cardiogenic shock.
Heart transplantation in peripartum cardiomyopathy
Early cardiac transplantation should be reserved for patients with refractory severe heart failure where MCS is not possible or not desirable for individual reasons, mainly for cases with biventricular failure or severe initial right ventricular dysfunction.
Stabilized/chronic heart failure
- For treatment of stabilized/chronic heart failure, the pregnancy status of the patient is important.
- Women who present with PPCM during pregnancy require joint cardiac and obstetric care.
- Possible adverse effects on the foetus must be considered when prescribing drugs.
- For those not breastfeeding, heart failure should be treated according to guidelines on acute and chronic heart failure including ACE inhibition, beta‐blockade and MRAs, and then replacing ACE inhibitors and ARBs with ARNI.
- As high resting heart rate is a predictor of adverse outcome, treatment with ivabradine might be useful in PPCM patients with high heart rate in sinus rhythm on top of beta‐blockade.
Delivery
- Vaginal delivery is always preferable if the patient is haemodynamically stable and there are no absolute obstetric indications for caesarean delivery.
- Close haemodynamic monitoring is required.
- Epidural analgesia is preferred.
- Urgent delivery irrespective of gestation duration should be considered in women with advanced heart failure and haemodynamic instability despite optimal heart failure treatment.
- In these cases, caesarean section is recommended with central neuraxial anaesthesia.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd