- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Engineers develop toy device to monitor respiration rate in asthmatic children
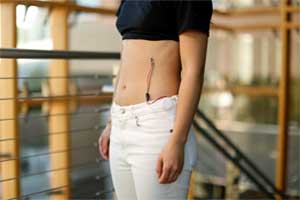
Researchers at the University of California, Irvine have developed a respiratory monitoring device using a toy, shrinky dinks, to help children who suffer from asthma and other pulmonary diseases. This new wearable device is inbuilt with disposable respiration sensors that track the rate and volume of a wearer's breath. The innovation was published in npj Digital Medicine.
Childhood asthma is characterized by inflammation of lungs and air duct when exposed to certain triggers. These triggers vary from child to child and can include: Viral infections such as the common cold, exposure to air pollutants, such as tobacco smoke, allergies to dust mites, pet dander, pollen or mould. Childhood asthma can cause bothersome daily symptoms that interfere with play, sports, school, and sleep. In some children, unmanaged asthma can cause dangerous asthma attacks. Childhood asthma isn't a different disease from asthma in adults, but children do face unique challenges. Asthma in children is a leading cause of emergency department visits, hospitalizations and missed school days.
This device will warn of an oncoming attack. The paired sensors one placed between the ninth and 10th ribs and the other on the abdomen track the rate and volume of the wearer’s respiration by measuring the local strain on the application areas
The devices are made by applying a very thin layer of metal to a sheet of the plastic toy and then heat-shrinking it to cause corrugation. The film is then transferred to a soft, stretchy material -- similar to small bandage -- that can be adhered to a patient. Signals from embedded sensors can be transmitted via Bluetooth to be displayed on a smartphone app.
Michelle Khine, UCI professor of biomedical engineering, in whose lab the devices were developed, said she was inspired to pursue the innovation after the birth of her son nine months ago. Complications required the newborn to be confined to the neonatal intensive care unit hooked up to an array of machines supplying oxygen and monitoring his breathing.
"Despite having his whole tiny body covered in sensors, all the hospital staff could get was respiration rate information. If you looked at the vitals monitor, you'd see this waveform, so it looked like they were getting [respiration volume] information, but they weren't," Khine said. "I felt so helpless with my child just lying in this box. I wasn't allowed to carry him for eight days, so it was heartbreaking -- but also frustrating to see all of these wires hooked up to him but not giving all the information we wanted."
Khine's lab is well-known for employing Shrinky Dinks as a platform for medical applications. About a decade ago, she innovated the use of the toy to produce microfluidic devices.
"It's amazing that this toy for kids has enabled us to create these robust sensors that may one day benefit children and others around the world," she said.
So far, members of the Khine lab have tested the new technology on healthy subjects, but there are plans for a pilot trial with a small number of asthma sufferers in the coming months.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd