- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Eczema drug restores hair growth in longstanding alopecia
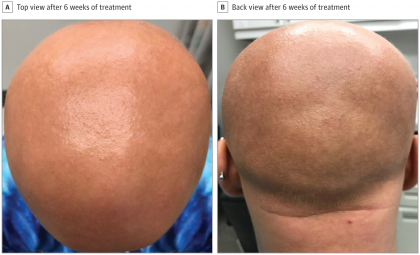
Maryanne M. Senna, Department of Dermatology, Massachusetts General Hospital, Boston, and colleagues in a case report published in the journal JAMA Dermatology, describe the case of a 13-year-old patient, who has alopecia totalis - a total lack of scalp hair - along with eczema, experienced significant hair regrowth following treatment with eczema drug dupilumab.
Dupilumab is FDA approved drug for the treatment of moderate to severe eczema, also called atopic dermatitis. It is marketed under the brand name Dupixent.

Credits: JAMA Dermatology
"We were quite surprised since this patient hadn't grown scalp hair since the age of 2, and other treatments that can help with hair loss did not in her case," says Dr. Senna. "As far as we know, this is the first report of hair regrowth with dupilumab in a patient with any degree of alopecia areata."
In addition to longstanding alopecia, this patient had experienced extensive, treatment-resistant eczema since the age of 7 months. Treatment with prednisone and methotrexate, medications that can suppress the overactive immune system, led to limited improvement in the patient's eczema but no hair regrowth and was therefore discontinued. In July 2017 she began to be treated with weekly injections of dupilumab, which had recently received FDA approval. After six weeks of treatment, which led to significant improvement in eczema symptoms, she also noticed that fine light hairs called vellus hairs were appearing on her scalp.
After seven months of dupilumab treatment, the patient had grown a significant amount of the pigmented hair that typically grows on the scalp. Because of a change in her insurance coverage, she had to discontinue dupilumab for a two-month period, during which she noticed shedding of the recently regrown hair. But after she could resume treatment in April 2018, the hair growth resumed and has continued.
Senna explains that dupilumab's mechanism of targeting a key immune system pathway known to be overactive in eczema could explain its action against alopecia since recent studies have suggested other elements of the same pathway may induce autoimmune hair loss. "Right now, it's hard to know whether dupilumab could induce hair growth in other alopecia patients, but I suspect it may be helpful in patients with extensive active eczema and active alopecia areata," she says. "We've submitted a proposal for a clinical trial using dupilumab in this patient population and hope to be able to investigate it further in the near future."
For further reference log on to 10.1001/jamadermatol.2018.2976

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd