- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Dupilumab beneficial in moderate-to-severe Atopic Dermatitis
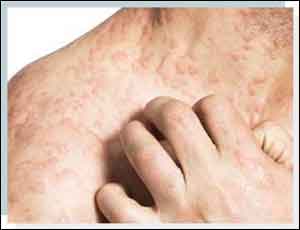
Dupixent (dupilumab) monotherapy resulted in significant improvements in the signs and symptoms of atopic dermatitis, according to the findings of Phase 3 trial presented at the recent European Academy of Dermatology and Venereology (EADV) Congress.
Dupixent is an interleukin-4 and interleukin-13 inhibitor and is currently approved to treat moderate-to-severe atopic dermatitis in adults whose disease is not adequately controlled with topical prescription therapies.
Regeneron Pharmaceuticals and Sanofi presented detailed results from a pivotal Phase 3 trial showing Dupixent®(dupilumab) monotherapy demonstrated a significant improvement in atopic dermatitis and a certain quality of life measures in adolescent patients (12-17 years) whose disease was inadequately controlled with topical therapies or for whom topical treatment was medically inadvisable.
"Limited treatment options leave adolescents with uncontrolled moderate-to-severe atopic dermatitis to cope with intense, unrelenting itch and skin lesions," said Amy S. Paller, principal investigator of the trial. "The results we are presenting today show the potential for Dupixent in adolescents to not only help clear the skin and reduce itching but also improve certain aspects of quality of life in adolescents who may be dealing with these unbearable symptoms."
The Phase 3 trial included 251 patients aged 12–17 years old with moderate-to-severe atopic dermatitis who were randomized into 3 groups for a control period of 16 weeks.
The first group was treated with Dupixent 200mg or 300mg every 2 weeks, based on weight (with an initial dose of 400mg or 600mg respectively). The second group was treated with Dupixent 300mg every 4 weeks (with an initial dose of 600mg). The third group was treated with placebo every 2 weeks. The primary endpoint was the proportion of patients with an Investigator's Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) at week 16.
Read Also:New Atopic Dermatitis Yardstick provides practical guidance and management insights
Key study findings:
- 41.5% of patients who received Dupixent every two weeks and 38% of patients who received Dupixent every four weeks achieved 75% or greater skin improvement (EASI-75) compared to 8% with placebo.
- 24% of patients who received weight-based dosing of Dupixent every two weeks (200 mg or 300 mg) and 18% of patients who received a fixed dose of Dupixent every four weeks (300 mg) achieved the primary endpoint – clear or almost-clear skin (IGA; score of 0 or 1) – compared with 2% with placebo.
- With regard to key secondary endpoints at 16 weeks, there was a 66% improvement in the Dupixent every two weeks group and 65% improvement in the Dupixent every four weeks group in average percent change from baseline in EASI score compared with a 24% improvement in the placebo group.
The overall rate of adverse events was 72% for Dupixent every two weeks, 64% for Dupixent every four weeks and 69% for placebo. Adverse events that were observed more frequently with Dupixent included injection site reactions and conjunctivitis.
Atopic dermatitis, a form of eczema, is a chronic inflammatory disease with symptoms often appearing as a rash on the skin. Moderate-to-severe atopic dermatitis is characterized by rashes that can potentially cover much of the body and can include intense, persistent itching, skin lesions, and skin dryness, cracking, redness, crusting and oozing.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd