- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Diacerein ointment to be future topical Treatment for Epidermolysis Bullosa
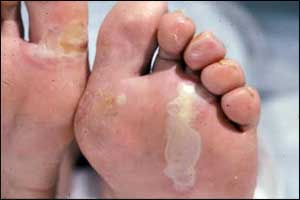
Diacerein 1% ointment (CCP-020) has been granted rare pediatric disease designation for the treatment of epidermolysis bullosa by The Food and Drug Administration (FDA) . Castle Creek Pharmaceuticals informed that 60% of patients treated with a diacerein cream formulation experienced at least a 40% reduction in blistering after four weeks of treatment.
Epidermolysis bullosa simplex (EBS) is the most common form of epidermolysis bullosa (EB), an uncommon dermatologic condition. For many patients, EBS can be debilitating and there is currently no treatment options available but only supportive care that includes wound care and use of bandages as well as pain management.
Diacerein 1% ointment (CCP-020) is a potentially disease-modifying investigational drug that may block an important inflammatory signaling pathway associated with EBS. Its efficacy and safety are now being evaluated in the DELIVERS clinical trial as a potential treatment for EBS. Diacerein is an anthraquinone with potent anti-inflammatory properties. CCP-020 is a formulation of diacerein developed specially for topical application; the product is currently being evaluated in a clinical trial (DELIVERS) in patients with epidermolysis bullosa simplex, a subtype of EB.
In a previously completed Phase 2 clinical trial that included 17 patients with EBS, 60% of patients treated with a diacerein cream formulation experienced at least a 40% reduction in blistering after four weeks of treatment, compared to 18% on the vehicle. During the study, adverse events (AE) occurred in 6 patients on diacerein versus 11 patients on the vehicle. The most notable AEs were increased in the blistering, pruritus and skin infection (1). However, none of the listed events were considered treatment-related or involved the treatment area.
Receiving this important rare pediatric disease designation is a significant recognition of CCP-020 and its potential as an important therapy for people living with EBS,” said Michael Derby, co-founder, chief executive officer, Castle Creek Pharmaceuticals.“We look forward to advancing the clinical development of CCP-020 in our ongoing DELIVERS study and fulfilling our mission to improve the health and quality of life for people living with EBS in the years ahead. We look forward to advancing the clinical development of CCP-020 in our ongoing DELIVERS study and fulfilling our mission to improve the health and quality of life for people living with EBS in the years ahead," said Michael Derby, CEO of Castle Creek Pharmaceuticals.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd