- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Cystic Fibrosis Drug Shows Promise in Children as Young as 1 Year of Age
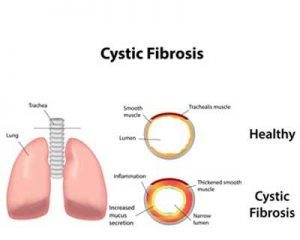
Ann & Robert H. Lurie Children’s Hospital of Chicago is one of the study sites in the open-label Phase 3 study that showed safety and effectiveness of the cystic fibrosis drug Kalydeco (ivacaftor) in children ages 1 to 2 years. Based on these results, Vertex Pharmaceuticals Inc., (Nasdaq: VRTX) plans to submit applications for the drug in this age group to the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Kalydeco is currently approved by the FDA for the treatment of cystic fibrosis in patients aged 2 years and older.
“Based on these new results and studies in older populations, we hope that early therapy that targets the underlying cause of the disease will promote longer and healthier lives,” said Susanna McColley, MD, Principal Investigator at Lurie Children’s, Associate Director of Cystic Fibrosis Center at Lurie Children’s, and Professor of Pediatrics at Northwestern University Feinberg School of Medicine.
Kalydeco treats the genetic cause of the cystic fibrosis – a defect in the CFTR gene. The children in the study had one of 10 mutations in this gene. Mutations in the CFTR gene lead to the insufficient flow of salt and water in and out of cells in a number of organs. In the lungs, this creates the buildup of thick, sticky mucus that can result in chronic lung infections, lung damage, and ultimately early death.
The current study, called ARRIVAL, met its primary endpoint of safety, with data that were consistent with findings from earlier Phase 3 studies of the drug in older children with cystic fibrosis. Results showed improvement in sweat chloride levels, which decreased substantially after 24 weeks of treatment, pointing to better CFTR function. The study also demonstrated improved measures of pancreatic function, which is important since pancreatic insufficiency is one of the most significant clinical manifestations of cystic fibrosis.
The study is ongoing in infants younger than 1 year.
“Our goal is to have therapies that can be given right after cystic fibrosis is diagnosed in babies, in the first month of life,” said McColley, who is also Associate Chief Research Officer for Clinical Trials at Stanley Manne Children’s Research Institute at Lurie Children’s.
Research at Ann & Robert H. Lurie Children’s Hospital of Chicago is conducted through the Stanley Manne Children’s Research Institute. The Manne Research Institute is focused on improving child health, transforming pediatric medicine and ensuring healthier futures through the relentless pursuit of knowledge. Lurie Children’s is ranked as one of the nation’s top children’s hospitals in the U.S. News & World Report. It is the pediatric training ground for Northwestern University Feinberg School of Medicine. Last year, the hospital served more than 198,000 children from 50 states and 51 countries.

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd