- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
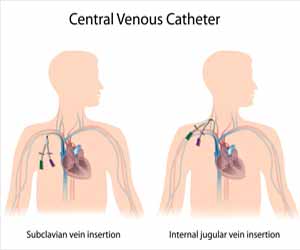
Central Venous Access: ASA 2020 Guidelines
Perform central venous catheterization in an environment that permits the use of aseptic techniques
Ensure that a standardized equipment set is available for central venous access
Use a checklist or protocol for placement and maintenance of central venous catheters
Use an assistant during placement of a central venous catheter
Do not routinely administer intravenous antibiotic prophylaxis
In preparation for the placement of central venous catheters, use aseptic techniques (e.g., hand washing) and maximal barrier precautions (e.g., sterile gowns, sterile gloves, caps, masks covering both mouth and nose, full-body patient drapes, and eye protection)
Use a chlorhexidine-containing solution for skin preparation in adults, infants, and children
For neonates, determine the use of chlorhexidine-containing solutions for skin preparation based on clinical judgment and institutional protocol
If there is a contraindication to chlorhexidine, povidone-iodine or alcohol may be used
Unless contraindicated, use skin preparation solutions containing alcohol
For selected patients, using catheters coated with antibiotics, a combination of chlorhexidine and silver sulfadiazine, or silver-platinum-carbon–impregnated catheters based on the risk of infection and anticipated duration of catheter use
Do not use catheters containing antimicrobial agents as a substitute for additional infection precautions
Determine catheter insertion site selection based on clinical need
Select an insertion site that is not contaminated or potentially contaminated (e.g., burned or infected skin, inguinal area, adjacent to tracheostomy or open surgical wound)
In adults, select an upper-body insertion site when possible to minimize the risk of infection
Determine the use of sutures, staples, or tape for catheter fixation on a local or institutional basis
Minimize the number of needle punctures of the skin
Use transparent bio occlusive dressings to protect the site of central venous catheter insertion from infection
Unless contraindicated, dressings containing chlorhexidine may be used in adults, infants, and children
For neonates, determine the use of transparent or sponge dressings containing chlorhexidine based on clinical judgment and institutional protocol
If a chlorhexidine-containing dressing is used, observe the site daily for signs of irritation, allergy or necrosis
Determine the duration of catheterization based on clinical need
Assess the clinical need for keeping the catheter in place on a daily basis
Remove catheters promptly when no longer deemed clinically necessary
Inspect the catheter insertion site daily for signs of infection
Change or remove the catheter when catheter insertion site infection is suspected
When a catheter-related infection is suspected, a new insertion site may be used for catheter replacement rather than changing the catheter over a guidewire
Clean catheter access ports with an appropriate antiseptic (e.g., alcohol) before each access when using an existing central venous catheter for injection or aspiration
Cap central venous catheter stopcocks or access ports when not in use
Needleless catheter access ports may be used on a case-by-case basis
Determine catheter insertion site selection based on clinical need and practitioner judgment, experience, and skill
Select an upper-body insertion site when possible to minimize the risk of thrombotic complications relative to the femoral site
Perform central venous access in the neck or chest with the patient in the Trendelenburg position when clinically appropriate and feasible
Select catheter size (i.e., outside diameter) and type based on the clinical situation and skill/experience of the operator
Select the smallest size catheter appropriate for the clinical situation
For the subclavian approach select a thin-wall needle (i.e., Seldinger) technique versus a catheter-over-the-needle (i.e., modified Seldinger) technique
For the jugular or femoral approach, select a thin-wall needle or catheter-over-the-needle technique based on the clinical situation and the skill/experience of the operator
For accessing the vein before threading a dilator or large-bore catheter, base the decision to use a thin-wall needle technique or a catheter-over-the-needle technique at least in part on the method used to confirm that the wire resides in the vein.
The number of insertion attempts should be based on clinical judgment
The decision to place two catheters in a single vein should be made on a case-by-case basis
Use real-time ultrasound guidance for vessel localization and venipuncture when the internal jugular vein is selected for cannulation.
When feasible, real-time ultrasound may be used when the subclavian or femoral vein is selected
Use static ultrasound imaging before prepping and draping for pre puncture identification of anatomy to determine vessel localization and patency when the internal jugular vein is selected for cannulation
Static ultrasound may also be used when the subclavian or femoral vein is selected
After insertion of a catheter that went over the needle or a thin-wall needle, confirm venous access.
Do not rely on blood colour or absence of pulsatile flow for confirming that the catheter or thin-wall needle resides in the vein
When using the thin-wall needle technique, confirm the venous residence of the wire after the wire is threaded
When using the catheter-over-the-needle technique, confirmation that the wire resides in the vein may not be needed (1) when the catheter enters the vein easily and manometry or pressure-waveform measurement provides unambiguous confirmation of the venous location of the catheter and (2) when the wire passes through the catheter and enters the vein without difficulty
If there is any uncertainty that the catheter or wire resides in the vein, confirm the venous residence of the wire after the wire is threaded; insertion of a dilator or large-bore catheter may then proceed.
After final catheterization and before use, confirm the residence of the catheter in the venous system as soon as clinically appropriate.
Confirm the final position of the catheter tip as soon as clinically appropriate
For central venous catheters placed in the operating room, perform a chest radiograph no later than the early postoperative period to confirm the position of the catheter tip
Verify that the wire has not been retained in the vascular system at the end of the procedure by confirming the presence of the removed wire in the procedural field
If the complete guidewire is not found in the procedural field, order chest radiography to determine whether the guidewire has been retained in the patient’s vascular system
When unintended cannulation of an arterial vessel with a dilator or large-bore catheter occurs, leave the dilator or catheter in place and immediately consult a general surgeon, a vascular surgeon, or an interventional radiologist regarding surgical or nonsurgical catheter removal for adults
For neonates, infants, and children determine on a case-by-case basis whether to leave the catheter in place and obtain consultation or to remove the catheter nonsurgically
After the injury has been evaluated and a treatment plan has been executed, confer with the surgeon regarding relative risks and benefits of proceeding with the elective surgery versus deferring surgery to allow for a period of patient observation
For further reference log on to :
Anesthesiology 1 2020, Vol.132, 8-43. doi:https://doi.org/10.1097/ALN.0000000000002864

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd