- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
BSG's latest Primary Biliary Cholangitis Guidelines
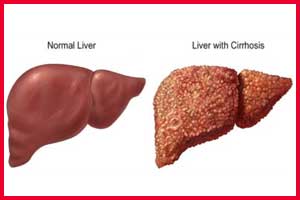
- Primary biliary cholangitis leads to a cycle of immune mediated biliary epithelial cell injury, cholestasis and progressive fibrosis can culminate over time in an end-stage biliary cirrhosis.
- It is most prevalent in women and those over the age of 50, but a spectrum of disease is recognised in adult patients globally; male sex, younger age at onset (<45) and advanced disease at presentation are baseline predictors of poorer outcome.
- Disease course is frequently accompanied by symptoms that can be burdensome for patients, and management of patients with PBC must address, in a life-long manner, both disease progression and symptom burden.
- Licensed therapies include ursodeoxycholic acid (UDCA) and obeticholic acid (OCA), alongside experimental new and re-purposed agents.
- Disease management focuses on initiation of UDCA for all patients and risk stratification based on baseline and on-treatment factors, including in particular the response to treatment.
- Those intolerant of treatment with UDCA or those with high-risk disease as evidenced by UDCA treatment failure (frequently reflected in trial and clinical practice as an alkaline phosphatase >1.67 × upper limit of normal and/or elevated bilirubin) should be considered for second-line therapy, of which OCA is the only currently licensed National Institute for Health and Care Excellence recommended agent.
- Follow-up of patients is life-long and must address treatment of the disease and management of associated symptoms.
UK-PBC and the British Society of Gastroenterology (BSG) have partnered to develop a comprehensive guideline document to provide detailed advice and recommendations on the best approaches to the management of the disease. A series of recommendations and audit standards are proposed to ensure that patients are offered timely licensed therapy (ursodeoxycholic acid (UDCA), obeticholic acid (OCA)) in addition to being actively managed for symptoms as well as complications of progressive liver disease.
Key recommendations, based on the GRADE classification system (Strong/Weak; quality of evidence: High/Moderate/Low/Very low), are:
The presence of antimitochondrial antibodies (>1 in 40) or highly PBC-specific antinuclear antibodies, in the appropriate context of cholestatic liver biochemistry, without alternative explanation, is usually sufficient for confidently reaching the diagnosis of PBC (Strong; High).
All patients with PBC should be offered structured life-long follow-up, recognising that different patients have different disease courses and may require different intensity of follow-up (Strong; Moderate).
Risk assessment should evaluate disease severity and activity at baseline and on treatment. We recommend a combination of serum liver tests (to identify those with an elevated bilirubin, a platelet count <150 or biochemical disease activity on treatment), imaging (liver ultrasound to identify overt cirrhosis and splenomegaly; transient elastography to identify increased liver stiffness) and recognition of young age at disease onset (<45 years) and male sex (Strong; Moderate).
To identify those at greatest risk of disease progression, we recommend that all patients have individualised risk stratification using biochemical response indices following 1 year of UDCA therapy (Strong; High). We suggest that UDCA treated patients with an alkaline phosphatase (ALP) >1.67 x upper limit of normal (ULN) and/or elevated bilirubin <2 x ULN represent a group of high-risk patients in whom there is randomised controlled trial evidence for the addition of second-line therapy (Weak; Moderate).
We recommend oral UDCA at 13–15 mg/kg/day is used as the first-line pharmacotherapy in all patients with PBC. If tolerated, treatment should usually be life-long (Strong; High).
In patients with inadequate response to UDCA (or UDCA intolerance) as defined by ALP >1.67 x ULN and/or elevated bilirubin <2 x ULN, the addition of OCA has been associated with improvements in biochemical surrogates of disease activity reasonably likely to predict improved outcomes. We therefore recommend, in keeping with the NICE evaluation of OCA, that the addition of OCA for patients with an inadequate response to UDCA, or intolerant of UDCA, is considered. We recommend dose adjustment in patients with advanced liver disease as per the drug label (Strong; Low).
We recommend all patients should be evaluated for the presence of symptoms, particularly fatigue and itch. Clinicians should recognise that severity of symptoms does not correlate with stage of disease (Strong; Moderate).
True overlap with autoimmune hepatitis is probably rare and we suggest that, when suspected, liver biopsy with expert clinicopathological review is needed to make the diagnosis and guide treatment (Strong; Moderate).
We recommend that patients with PBC should be offered the chance to seek support from patient support groups (Strong; Moderate).
We recommend that clinicians caring for patients with PBC should consider introducing clinical audit tools to document and improve the quality of care delivered to patients (Strong; Low).
For further reference log on to :
http://gut.bmj.com/content/early/2018/03/28/gutjnl-2017-315259

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd