- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Bladder Cancer-Standard Treatment Guidelines
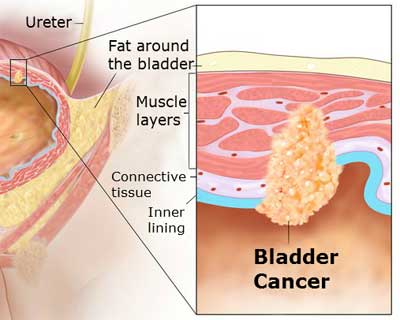
Introduction
Ministry of Health and Family Welfare, Government of India has issued the Standard Treatment Guidelines for Bladder Cancer.
Following are the major recommendations :
Case definition
Bladder carcinoma is the most common malignancy of the urinary tract and is the 9th most common cancer diagnosis worldwide. At the initial diagnosis of bladder cancer, 70% of cases are diagnosed as non-muscle-invasive bladder cancer (NMIBC) and approximately 30% as muscle-invasive disease. [1, 2] Non-muscle-invasive bladder cancer: Bladder cancer that does not involve the muscularis propria.
Invasive bladder cancer: Bladder cancer that histologically invades the muscularis propria.
• Risk factors [3,4,5,6]
– Tobacco consumption
– Occupational exposure to chemicals
– Radiation therapy
– Chronic urinary tract infection
– Bladder schistosomiasis
– Chemotherapy – Cyclophosphamide
• Active and passive tobacco smoking continues to be the main risk factor, while
exposure-related incidence is decreasing.
INCIDENCE OF THE CONDITION IN OUR COUNTRY:
Exact incidence is unknown. The recent trends indicate increasing incidence of bladder cancer. This may be partially attributed due to better detection and improved health care. Expected to be of same incidence as the western world.
DIFFERENTIAL DIAGNOSIS
- Chronic cystitis
- Tuberculous cystitis
- Bladder calculi
- Interstitial cystitis
- Radiation cystitis
- Eosinophilic cystitis
PREVENTION AND COUNSELING
- Mass education about bladder cancer and its relationship with tobacco use
- Anti-tobacco campaign
- Careful history about smoking, occupational exposure to risk factors and
storage LUTS - Detailed evaluation of all patients with gross hematuria and elderly patients
(>40 years) with microscopic hematuria and associated risk factors like
smoking - All patients with hematuria should undergo full urological evaluation
- Prompt referral of men with advanced bladder cancer to higher centers for
further evaluation
OPTIMAL DIAGNOSTIC CRITERIA, INVESTIGATIONS, TREATMENT & REFERRAL CRITERIA
Diagnostic criteria:
1. History of gross painless hematuria
2. History of severe storage LUTS – may be due to CIS
3. Recurrent cystitis in elderly
4. Positive cytology
5. Cystoscopic examination and imaging studies showing tumour(s) in the
bladder.
Diagnosis –The diagnosis mainly depends on the cystoscopic examination of the
bladder, biopsy, and urine cytology. The initial therapy for bladder tumours is complete macroscopic transurethral resection of bladder tumours (TURBT) including a part of underlying muscle [7]. Cold cup biopsies should be discouraged.
A second TURBT should be considered [8]:
1. If there is suspicion that the initial resection was incomplete
2. When multiple or large tumours are present
3. When pathologist reported no muscle in the specimen
4. When a high grade tumour (pT1G3) was detected.
The management algorithm is based on the diagnosis of invasion of muscularis propria or not.
Routine bladder mapping biopsies are not indicated except in
1. Patients with positive urine cytology with normal looking mucosa in cystoscopy
2. Biopsy of the apical prostatic urethra when there is a bladder neck tumour or when
abnormalities of prostatic urethra are visible.
Carcinoma in situ (CIS) is diagnosed based on the histology of bladder mucosal biopsies.
Fluorescent cystoscopy is recommended in these cases [9].
Investigations:
- Urine cytology: Cytology is useful when a high-grade malignancy or CIS is present. It is used to predict high grade tumour before TUR. However, urinary cytology often is negative in the presence of low-grade cancer.
- Ultrasonography (USG): Transabdominal USG permits characterization of renal masses, detection of hydronephrosis and visualization of intraluminal masses in the bladder. It can be as accurate as IVU for diagnosis of upper urinary tract obstruction [10]. The USG is thus a useful tool for investigation in patients with haematuria to detect obstruction; it cannot however exclude the presence of upper tract tumours.
- Pelvic examination (Bimanual examination) under anaesthesia: Helpful in assessment of local staging in muscle invasive bladder cancer and advanced cases. Not of much value in superficial bladder cancers. Should be done along with TURBT.[11]
- Cystoscopy & TURBT: Cystoscopy should describe all macroscopic features of the tumour (site, size, number and appearance) and mucosal abnormalities. A bladder diagram is recommended. The gold standard in establishing the diagnosis of bladder tumour is TURBT.
- Intravenous Urography (IVU): Intravenous urography (IVU) is used to detect filling defects in the calyces, renal pelvis and ureters, and hydronephrosis. Acceptable for staging of muscle invasive bladder cancer when CT Urography is not readily available [12].
- CT Urography (CTU): CTU is mainly recommended for histologically proven muscle invasive bladder cancers for staging. It is not useful for making a diagnosis of muscle invasive bladder cancer. Pre TURBT CTU is indicated in select group of patients in whom it would significantly alter the management. Especially in muscle invasive tumours of the bladder and in upper tract tumours, CT urography gives more information than IVU does (including status of lymph nodes and neighbouring organs) [12].
- MRI – abdomen and pelvis/ MR Urography: Optimal investigation for staging in muscle invasive bladder cancer Recommended only when there is definite contraindication for CT urography or IVU like contrast allergy and renal failure.
- Serum alkaline phosphatase – if elevated indicates metastatic bone disease.
- Bone scan –Indicated in patients with raised alkaline phosphatase and with bone pain.
- CT scan of chest is recommended for optimal staging in muscle invasive bladder cancer; if not available chest X-ray is acceptable.
Staging of bladder cancer:
Based on Tumour Node Metastasis (TNM) classification of carcinoma bladder (2010)
• T - primary tumour
• TX Primary tumour cannot be assessed
• T0 No evidence of primary tumour
• Ta Non-invasive papillary carcinoma
• Tis Carcinoma in situ. ‘flat tumour’
• T1 Tumour invades subepithelial connective tissue
• T2 Tumour invades muscle
• T2a Tumour invades superficial muscle (inner half)
• T2b Tumour invades deep muscle (outer half)
• T3 Tumour invades perivesical tissue
• T3a Macroscopically
• T3b Microscopically (extravesical mass)
• T4 Tumour invades any of the following: Prostate, uterus, vagina, pelvic wall,
abdominal wall
• T4a Tumour invades prostate, uterus or vagina
• T4b Tumour invades pelvic wall or abdominal wall
• N - regional lymph nodes
• NX Regional lymph nodes cannot be assessed
• N0 No regional lymph node metastasis
• N1 Metastasis in a single lymph node in the true pelvis (hypogastric, obturator,
external iliac or presacral)
• N2 Metastasis in a multiple lymph nodes in the true pelvis (hypogastric,
obturator, external iliac or presacral)
• N3 Metastasis in a common iliac lymph node(s)
• M - distant metastasis
• MX Distant metastasis cannot be assessed
• M0 No distant metastasis
• M1 Distant metastasis
Characteristics of Stages Ta, T1, and Tis
Stage Ta tumours are confined to the urothelium, have a papillary configuration of their exophytic part, and do not penetrate from the urothelium into the lamina propria or detrusor muscle.
Stage T1 tumours originate from the urothelium but penetrate the basement membrane which separates the urothelium from the deeper layers. T1 tumours invade into the lamina propria, but are not so deep that they reach the detrusor muscle.
Carcinoma in situ (Tis) is a high-grade (anaplastic) carcinoma confined to the urothelium, but with a flat non-papillary configuration. Unlike a papillary tumour, Tis appears as reddened and velvety mucosa and is slightly elevated but sometimes not visible. Tis can be local or diffuse.
Three types of Tis are distinguishable;
- Primary Tis (no previous or concurrent papillary tumours)
- Secondary Tis (with a history of papillary tumours)
- Concurrent Tis (in the presence of papillary tumours).
Characteristics of grade [14]:
1973 WHO Classification
Apart from their architecture, the individual cells show different degrees of anaplasia:
Grade 1: well differentiated tumour
Grade 2: moderately differentiated tumour
Grade 3: poorly differentiated tumour
2004 WHO Classification
A new classification system was initially proposed by the WHO/ISUP in 1998 and
updated by the WHO in 2004. For non-invasive urothelial neoplasias, the categories are:
• Flat lesions
• Hyperplasia (flat lesion without atypia or papillary)
• Reactive atypia (flat lesion with atypia)
• Atypia of unknown significance
• Urothelial dysplasia
• Urothelial carcinoma in situ (CIS)
• Papillary lesions
• Urothelial papilloma (a completely benign lesion)
• Papillary urothelial neoplasm of low malignant potential
• (PUNLMP)
• Low-grade papillary urothelial carcinoma
• High-grade papillary urothelial carcinoma
The 2004 WHO grading system defines Tis as a non-papillary, i.e. a flat, lesion in which the surface epithelium contains cells that are cytologically malignant. Papillary tumours are classified as either papillary urothelial neoplasms of low malignant potential (PUNLMP) or as urothelial carcinomas, with the latter being subdivided into two grades: low grade and high grade. The intermediate group (G2) has been eliminated; this group was the subject of controversy in the 1973 WHO classification. Use of the 2004 WHO classification is advocated, as this should result in less diagnostic variability among pathologists.
Predicting recurrence and progression of tumours [15,16]:
TaT1 tumours
The pattern of recurrence and progression depends on the following clinical and
pathological factors:
1. Number of tumours
2. Tumour size
3. Prior recurrence rate
4. T-category
5. Pesence of concurrent CIS
6. Tumour grade.
CIS
No prognostic factors are well established. Retrospective studies suggest the following:
1. Concurrent CIS with T1 tumours have worser prognosis than primary CIS and
secondary CIS [17, 18]
2. Responders to BCG have better prognosis than those non-responders. [19]
Treatment: Treatment strategy varies according to the stage and grade of bladder cancer.
Non-muscle invasive bladder cancer (superficial bladder cancer) NMIBC:
The standard initial therapy for Ta and T1 papillary bladder tumours is complete TURBT. Tumours less than 1cm in size can be resected enbloc. Larger tumours should be resected in fractions, which include the exophytic part, the underlying bladder wall and the edges of resection area. Complete and correct TUR is essential to achieve a good prognosis [20].
Prognostic Factors and Adjuvant Treatment
TaT1 papillary tumours
Recommendations for Low Risk Tumours
Patients with a single, small, low grade Ta tumour without CIS, who are at low risk for both recurrence and progression, should receive:
1. A complete TUR.
2. An immediate single post-operative instillation with a chemotherapeutic agent (drug optional – Mitomycin C preferred). [21]
3. No further treatment is recommended prior to recurrence.
Recommendations for High Risk Tumours
Patients with TaT1 high grade tumours with or without CIS and those with CIS alone are at high risk of progression. Treatment should consist of:
1. Complete TUR of papillary tumours followed by an immediate post-operative
instillation with a chemotherapeutic agent (drug optional – Mitomycin C preferred).[21]
2. A second TUR after 4–6 weeks.[9]
3. Adjuvant intravesical immunotherapy with BCG (full dose or reduced dose in case of
side effects). Maintenance therapy for at least 1 year (monthly once) is necessary
[22,23] although the optimal maintenance scheme has not yet been determined.
4. Immediate cystectomy may be offered to patients at highest risk of tumour of
progression (Patients with multiple tumours, large tumours (> 3 cm), and highly
recurrent tumours (> 1 recurrence/year), stage T1 tumours with high grade tumours,
and CIS).
5. In patients with BCG failure, cystectomy is recommended. [24]
Recommendations for Intermediate Risk Tumours
In the remaining intermediate risk patients, adjuvant intravesical therapy is necessary but no consensus exists regarding the optimal drug and the most appropriate scheme. BCG is more effective than chemotherapy in both reducing recurrence and progression. The major issue in the management of intermediate risk tumours is to prevent recurrence and progression, of which recurrence is clinically the most frequent.
Treatment should include:
- Complete TUR followed by an immediate postoperative instillation with a chemotherapeutic agent (drug optional).
- A second TUR after 4–6 weeks when the initial resection was incomplete.
- Adjuvant intravesical chemotherapy (drug optional), schedule: optional although the duration of treatment should not exceed 1 year. (Or)
- Adjuvant intravesical immunotherapy with BCG (full dose or reduced dose in case of side effects). Maintenance therapy for at least 1 year (monthly once) is necessary although the optimal maintenance schedule has not yet been determined.
Carcinoma in situ
CIS have a high risk of progression to muscle invasive disease which exceeds 50% in some studies. BCG intravesical immunotherapy (induction and maintenance) is superior to intravesical chemotherapy in increasing the complete response rate and the overall percent of patients remaining tumour free. Moreover, BCG reduces the risk of progression as compared to either intravesical chemotherapy or a different immunotherapy [25]. Early radical cystectomy at the time of diagnosis provides excellent disease-free survival, but over-treatment occurs in up to 50% of patients.
Recommendations for the treatment of CIS
- In concurrent CIS, the initial strategy (TUR, early intravesical instillation, a second TUR) is based on the features of the papillary tumour.
- Intravesical BCG immunotherapy including at least 1 year maintenance.
- After the 6 week induction course, a second course of 6 weekly BCG instillations or maintenance cycles consisting of 3 weekly instillations may be considered in nonresponders since about 40-60% of these patients will respond to additional treatment with BCG. [23]
- In BCG non-responders at 6 months, radical cystectomy is recommended. [26].
Muscle invasive bladder cancer:
Neo-adjuvant chemotherapy:
Neo-adjuvant cisplatin-containing combination chemotherapy improves overall survival by 5-7% at 5 years [27]. It should be considered irrespective of the type of definitive treatment. Neo-adjuvant chemotherapy is not recommended in patients with performance status (PS) > 2 and impaired renal function [28].
Radical Surgery and Urinary Diversion
Cystectomy is the preferred curative treatment for localized muscle invasive bladder cancer [29].
Radical cystectomy includes removal of regional lymph nodes, the extent of which has not been sufficiently defined [30]. A delay in cystectomy increases the risk of progression and cancer-specific death [31]. No pre-operative radiotherapy should be administered. Radical cystectomy in both sexes must not include the removal of the entire urethra in all cases, which may then serve as outlet for an orthotopic bladder substitution. If no bladder substitution is attached, the urethra must be checked regularly. Terminal ileum and colon are the intestinal segments of choice for urinary diversion. The type of urinary diversion does not affect oncological outcome.
Contraindications for orthotopic bladder substitution [32]:
1. Positive margins at the level of urethral dissection
2. Positive margins anywhere on the bladder specimen (in both sexes), if the primary tumour is located at the bladder neck or in the urethra (in women), or if tumour extensively infiltrates the prostate.
Pre-operative bowel preparation is not mandatory.
Before cystectomy, the patient should be counselled adequately regarding all possible alternatives, and the final decision should be based on a consensus between patient and surgeon.
For patients with inoperable locally advanced tumours (T4b), primary radical cystectomy is a palliative option and not recommended as a curative treatment.
Neoadjuvant Radiotherapy in Muscle-Invasive Bladder Cancer [33]
Pre-operative radiotherapy does not increase the survival for operable muscle invasive bladder cancer.
Bladder-Sparing Treatments
Radical TURBT
Radical TURBT is not recommended except in a rare situation when patient not willing for open surgery or unfit for radical cystectomy [34].
External beam radiotherapy [35, 36]
External beam radiotherapy alone should only be considered as a therapeutic option when the patient is unfit for cystectomy or a multimodality bladder-preserving approach Radiotherapy can also be used to stop bleeding from the tumour when local control cannot be achieved by transurethral manipulation because of extensive local tumour growth.
Chemotherapy [37,38]
Although cisplatin-based chemotherapy, as primary therapy for locally advanced tumours in highly selected patients, has led to complete and partial local responses, the long-term success rate is low.
Multimodality treatment [39,40]
There are comparable long-term survival rates in cases of multimodality treatment success. Delay in surgical therapy can compromise survival rates.
Adjuvant Chemotherapy [41]
Adjuvant chemotherapy is advised within clinical trials, but not for routine use.
Metastatic Disease [42 -47]
Urothelial carcinoma is a chemosensitive tumour. Performance status (PS) and the presence or absence of visceral metastases are independent prognostic factors for survival.
These factors are at least as important as the type of chemotherapy administered. Cisplatin-containing combination chemotherapy is able to achieve a median survival of up to 14 months, with long-term disease-free survival reported in about 15% of patients with nodal disease and good PS. Single-agent chemotherapy provides low response rates of usually short duration. Post-chemotherapy surgery after a partial or complete response may contribute to long-term disease-free survival. Prognostic factors should guide the treatment selection.
NOTE : All chemotherapeutic drugs/ targeted therapy should be decided and administered in consultation with a Urologist.
First-line treatment for “fit” patients:
Use cisplatin-containing combination chemotherapy with GC, MVAC, preferably with GCSF, or HD-MVAC with GCSF.
Carboplatin and non-platinum combination chemotherapy is not recommended.
First-line treatment in patients ineligible (‘unfit”) for cisplatin:
Use carboplatin combination chemotherapy or single agents. For cisplatin-ineligible patients (‘unfit’) with either PS 2 or impaired renal function, or with poor prognostic factors, first-line treatment is carboplatin-containing combination chemotherapy, preferably with gemcitabine/carboplatin
Second-line treatment:
In patients progressing after platinum-based combination chemotherapy for metastatic disease vinflunine should be offered, which has the highest level of evidence to date, or clinical trials of other treatments.
Follow-up for non-muscle invasive bladder tumours [48, 49]
Patients with non-muscle invasive bladder tumours need to be regularly followed up because of the risk of recurrence and progression; however, the frequency and duration of cystoscopies should reflect the individual patient’s degree of risk. The result of the first cystoscopy after TUR at 3 months is a very important prognostic factor for recurrence and for progression. The first cystoscopy should thus always be performed at 3 months after TUR in all patients with non-muscle invasive bladder tumour.
Recommendations for follow-up cystoscopy
Patients with tumours at low risk of recurrence and progression should have a cystoscopy at 3 months. If negative, the following cystoscopy is advised at 9 months and consequently yearly for 5 years.
Patients with tumours at high risk of progression should have a cystoscopy and urinary cytology at 3 months. If negative, the following cystoscopies and cytologies should be repeated every 3 months for a period of 2 years, every 4 months in the third year, every 6 months thereafter until 5 years, and yearly thereafter. A yearly evaluation of the upper tract by IVU or retrograde pyelogram is recommended.
Patients with intermediate-risk of progression (about one-third of all patients) should have an in-between follow-up scheme using cystoscopy and cytology, adapted according to personal and subjective factors.
Patients with Tis should be followed up for life due to the high risk of recurrence and progression, both within the bladder and extravesically. Urine cytology together with cystoscopy (and bladder biopsies in cytology positive cases) is essential for monitoring of treatment efficacy. The follow-up schedule is the same as for patients with high-risk tumours.
Follow up for muscle invasive bladder cancer [50]
Follow-up is based on the stage of initial tumour after cystectomy. At every visit, the following should be performed:
History, Physical examination, Serum chemistries and chest radiograph annually for pT1 disease; semiannual evaluation for patients with pT2 disease; and quarterly evaluation for patients with pT3 disease. For the last group, semiannual CT scan is recommended. Bone scan only when indicated or symptomatic. IVU can be done for upper tract surveillance when CT scan is not readily available. After 5 years of follow-up, stop oncological surveillance and continue with functional surveillance.
Referral criteria:
- Patients with gross painless hematuria with association with high risk factors.
- Patients with severe storage LUTS with or without palpable pelvic mass suggestive of bladder cancer.
- Those with advanced bladder cancer and metastases.
Situation 1: At Secondary Hospital / Non-Metro Situation: Optimal Standards Of Treatment In Situations Where Technology And Resources Are Limited.
Clinical diagnosis: Careful evaluation of patients with gross painless hematuria, Pelvic examination for bladder masses, not useful in non-muscle invasive bladder cancers.
Investigations: Urine microscopy for hematuria, urine cytology for malignant urothelial cells. Ultrasound of abdomen and pelvis - to localize the cause of hematuria like renal and bladder tumours. Cystoscopy in all cases of gross hematuria and those with bothersome severe storage LUTS and positive microscopic hematuria.
Treatment: According to the stage of the disease.
Standard operating procedure:
- Inpatient:
- Outpatient:
- Daycare:
Referral criteria:
Patients with gross painless hematuria known to have bladder tumour
Positive urine dipstick or microscopic hematuria
Positive urine cytology
Situation 2: At super specialty facility in metro location where higher end technology is available.
Clinical diagnosis: Evaluation of gross hematuria, positive microscopic hematuria and positive urine cytology. Pelvic examination – to look for bladder masses.
Investigations: All the possible investigations mentioned.
Treatment: According to the stage of the disease and the treatment options selected by the patient after counseling.
Standard operating procedure
- Inpatient
- Outpatient
- Daycare
Guidelines by The Ministry of Health and Family Welfare :
Prof. Rajesh Ahlawat
Chairman,
Department of Urology & Kidney Transplant,
Medanta Medicity
Reviewed By:
Dr Anup Kumar Gupta
Head of Department
Department of Urology
VMMC and Safdarjang Hospital,
New Delh

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd