- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
Antibiotic impregnated shunts reduce risk of shunt infection in hydrocephalus: Lancet
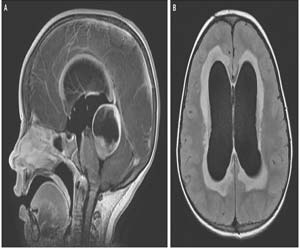
Ventriculoperitoneal shunts are one of the commonest tools used in the treatment of hydrocephalus. However, the risk of shunt infection affects up to 15% of hydrocephalus patients. In a recent BASICS trial, scientists have figured out a way to minimize the chances of shunt Infection. The trial reaved that antibiotic (rifampicin, and clindamycin) impregnated shunts are effective in reducing the risk and harm of shunt infection in hydrocephalus.
The authors have used sliver impregnated shunts and rifampicin, and clindamycin impregnated shunts and compared the chances of infection with the standard shunts. They found that rifampicin and clindamycin shunts have higher efficacy in providing protection against infection as compared to sliver and standard shunts. The study was published recently in Lancet
The authors conducted a parallel, multicentre, single-blind, randomized controlled trial, which included patients with hydrocephalus of any etiology undergoing insertion of their first ventriculoperitoneal shunt irrespective of age at 21 regional adult and pediatric neurosurgery centers in the UK and Ireland.
The authors randomly assigned patients (1:1:1 in random permuted blocks of three or six) to receive standard shunts (standard shunt group), antibiotic-impregnated (0·15% clindamycin and 0·054% rifampicin; antibiotic shunt group), or silver-impregnated shunts (silver shunt group) through a randomisation sequence generated by an independent statistician. All patients and investigators who recorded and analyzed the data were masked for group assignment, which was only disclosed to the neurosurgical staff at the time of operation. Participants receiving a shunt without evidence of infection at the time of insertion were followed up for at least 6 months and a maximum of 2 years. The primary outcome was time to shunt failure due to the infection and was analyzed with Fine and Gray survival regression models for competing for risk by intention to treat.
Key Findings
- 1594 had a shunt inserted without evidence of infection at the time of insertion (533 in the standard shunt group, 535 in the antibiotic shunt group, and 526 in the silver shunt group) and were followed up for a median of 22 months.
- 32 of 533 evaluable patients in the standard shunt group had a shunt revision for infection, compared with 12 of 535 evaluable patients in the antibiotic shunt group and 31 of 526 patients in the silver shunt group.
- 135 patients in the standard shunt group, 127 in the antibiotic shunt group, and 134 in the silver shunt group had adverse events, which were not life-threatening and were mostly related to valve or catheter function.
Results of the study made the authors conclude that "The BASICS trial provides evidence to support the adoption of antibiotic shunts in UK patients who are having their first ventriculoperitoneal shunt insertion. This practice will benefit patients of all ages by reducing the risk and harm of shunt infection."
For more details, click on the link

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd