- Home
- Editorial
- News
- Practice Guidelines
- Anesthesiology Guidelines
- Cancer Guidelines
- Cardiac Sciences Guidelines
- Critical Care Guidelines
- Dentistry Guidelines
- Dermatology Guidelines
- Diabetes and Endo Guidelines
- Diagnostics Guidelines
- ENT Guidelines
- Featured Practice Guidelines
- Gastroenterology Guidelines
- Geriatrics Guidelines
- Medicine Guidelines
- Nephrology Guidelines
- Neurosciences Guidelines
- Obs and Gynae Guidelines
- Ophthalmology Guidelines
- Orthopaedics Guidelines
- Paediatrics Guidelines
- Psychiatry Guidelines
- Pulmonology Guidelines
- Radiology Guidelines
- Surgery Guidelines
- Urology Guidelines
A new, portable device to detect TB in the offing
A new, portable device to detect TB in the offing By Susheela Srinivas
Tuberculosis is a highly contagious and fatal disease, and it is currently diagnosed by a DNA-based test called NAAT, short for Nucleic Acid Amplification Test. However, it is unreachable to a vast population especially in remote and backward regions, as the equipment is expensive and is infrastructure dependent.
A team of two researchers, Dr Bhushan J Toley and his PhD scholar Navjot Kaur from the Indian Institute of Science, Bengaluru, have now developed a compact, stand-alone and portable device that promises to overcome the limitation by drastically cutting down the costs.
The new device, which is also based on the concept of nucleic acid amplification, works on a method called LAMP detection or Loop-Mediated Isothermal Amplification of the DNA of the TB bacteria present in infected samples. The screening uses a fluorescence reporter material that latches on to the amplified bacterial DNA. It has been named FLIPP-NAAT, short for Fluorescent Isothermal Paper-and-Plastic NAAT. It has a ‘blister-pack’ type assembly which can be stacked, transported and stored conveniently, especially in TB surveillance vehicles.
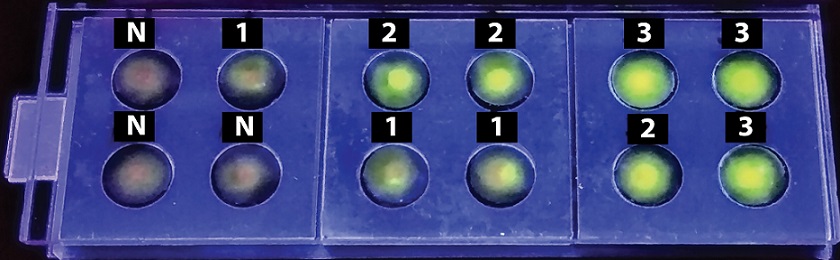
The readings are taken by using a low-cost UV torch to detect fluorescence response and is coupled to a mobile camera to capture the images. The scientists' duo along with co-author Dr Joy S Michael from the Christian Medical College, Vellore have performed a ‘blind test’ on 30 clinical samples. The device demonstrated its efficacy with 100% sensitivity in infected samples.
“The FLIPP-NAAT device is an assembly of multiple layers of acrylic sheets bonded together by a double-sided pressure-sensitive adhesive. The layers are designed to create cavities, within which tiny glass fibre discs are embedded. The reaction for the detection of tuberculosis DNA occurs within these paper discs, which has dried reagents and fluorescence material,” explained Dr Toley, while speaking to India Science Wire.
The DNA samples of the sputum are placed in the discs and incubated for about an hour. The top layer is then peeled off and the fluorescent material is added and resealed. The UV light activates green – coloured fluorescence in the infected samples, and the mobile camera captures the images.
“ Two tests were done on each sample to increase the reliability: The samples were compared and correlated with a known degree of bacterial DNA (called positive control) and another without the disease condition (called the negative control),” clarified Navjot Kaur, first author of the study.
The images from the camera are coupled to a widely used quantitative software which gives the degree of infection.
“Prompt and precise diagnosis in presumptive TB individuals is the need of the hour for treatment and prevention of the disease. The FLIPP-NAAT is a novel initiative in that line“, commented Dr Shanmugam Sivakumar, Scientist from the ICMR - National Institute for Research in Tuberculosis, Chennai, who was not involved in the study.
“Although the sensitivity is good, the device requires further refinement to improve the specificity (which is now 68.75%) and additional validation with more clinical samples before it is made available as a point of care test,” he added.
Dr Toley said, “We are working on several strategies to improve the specificity of the test to reduce the rate of false-positive results”.
The team is scaling the work to extend the shelf life of the dry reagents, along with developing a low-cost portable method to extract DNA from sputum samples. An app-based readout is also on the cards.
The results were published in Scientific Reports
India Science Wire

Disclaimer: This site is primarily intended for healthcare professionals. Any content/information on this website does not replace the advice of medical and/or health professionals and should not be construed as medical/diagnostic advice/endorsement or prescription. Use of this site is subject to our terms of use, privacy policy, advertisement policy. © 2020 Minerva Medical Treatment Pvt Ltd